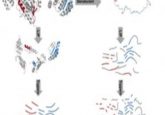
Time to die: instructing injured axons to degenerate
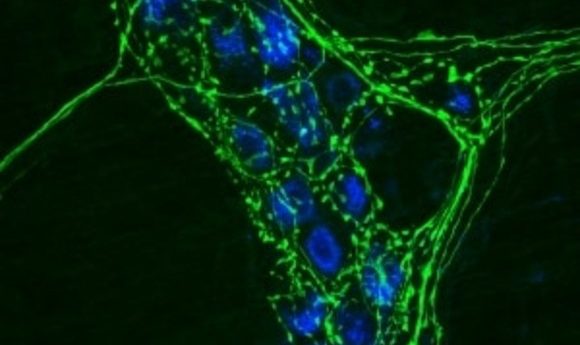
Researchers have found the first receptor that mediates axon death after an injury.
Mouse neurons with axons stained in green.
Credit: Wikipedia, Swharden
Early in an animal’s development, its nervous system undergoes a frenetic phase of building. Many more neurons, axons, and synapses are formed than the adult organism needs. These excess parts are eventually pared down as cells and axons die in response to developmental cues.
While this cell death is normal—and necessary—for the animal’s growth and function, neurons can also die as a result of injury or neurodegenerative disease. This neuronal cell death is thought to result from passive intrinsic mechanisms rather than receptors that respond to external signals. For the first time, however, researchers led by Christopher Deppman of the University of Virginia report in Current Biology that an orphan receptor named DR6 is required to induce axonal death after an injury.
DR6 is a member of the tumor necrosis factor receptor (TNFR) family of receptors, which are known to be critical to pruning axons during development. “We know many of the receptors and signaling pathways involved in developmental death, but no receptors have ever been discovered for injury,” Deppmann said. “People just assumed that these are totally independent processes. The premise that we started with essentially challenges that.”
The team began by testing if various TNFR family members played a part in injury-induced axon death. Using a microfluidic model where axons and nerve cell bodies could be studied in separate compartments, they grew neurons from mouse strains that lacked one or more of the TNFR family receptors (DR6, p75NTR, and TNFR1a) as well as control animals. In the absence of a nerve growth factor, wild-type axons degenerated completely within 24 hours, but mutants lacking DR6 only degenerated half as much as controls. When cultured axons were cut, those lacking DR6 remained intact up to a day later, suggesting that DR6 is necessary for injured axons to die off. “DR6 is not one of the classic receptors involved in developmental degeneration, although it does play some roles there,” Deppman said. “But it’s highly related to all the other family members that have been involved in developmental degeneration.”
The researchers found similar effects when they severed the sciatic nerve axon in wild-type and DR6 mutant mice. They also found a surprising effect of DR6 on the myelin sheath, a protective fatty layer that surrounds axons. In the absence of this receptor, uninjured axons were larger than wild-type ones. But after injury, mutants lacking DR6 showed a higher proportion of axons with aberrant myelin sheaths.
“Previously, axon degeneration and myelin remodeling were considered part of the same process—when an axon dies, the myelin wrapped around it receives the same signal, and they die together,” said Kanchana Gamage, a graduate student in Deppmann’s lab and first author of the article. “But from what we found, these are distinct processes.”
The results suggest that signaling pathways that evolved for nervous system development may have been repurposed to respond to signals during injury, according to Deppmann. They also reveal that axons’ communication with one another or neighboring Schwann cells—a common occurrence during development—may be important during neurodegenerative or injury-induced death.
Although it’s clear that the DR6 receptor responds to an external signal, precisely which signal is still a mystery. In future studies, the team aims to identify this cue and its source, whether from neighboring axons or supporting cells in the nervous system.
Receptors for injury-linked signals could play crucial roles in the pathology of traumatic brain injury, stroke, or neurodegenerative conditions such as Alzheimer’s disease, where “a small area of neurons are injured but a huge halo of bystander cells are affected as a result,” Deppmann said. “Finding the mechanisms that coordinate this process has broad implications for many conditions.”