Lighting up the inside
Glow-in-the-dark mice shed light on the mysteries of brain damage.
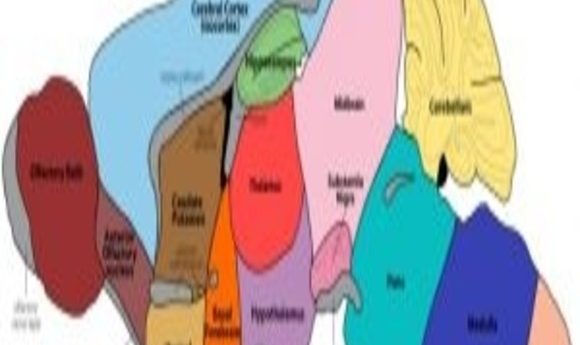
Experimental models are valuable tools for investigating Alzheimer’s Disease, Huntington’s Chorea, and Parkinson’s Disease, but direct observation of patient’s brains presents particular challenges to researchers. In part, this is due to the nature of the brain and the fact that its function across time is as important as its static condition at any one moment. Therefore, a snapshot from a CT scan or frozen slice tells only part of the story of biological processes in the brain.
Neurogenesis is the process by which the brain regrows neurons following disease or injury, and it is of great importance to researchers seeking to understand how the brain can self-repair. But recording this activity requires a living subject and the imaging must be in vivo, thus limiting the techniques available to neuroscientists. Using an innovative repurposing of insect bioluminescence, researchers now present a way to circumvent these obstacles and image the brain over long period of time in living subjects.
Enter the firefly, member of the family Lampyridae and producer of a class of compounds called luciferins. As their name suggests, luciferins emit light when broken down by the enzyme luciferase. These compounds offer researchers a noninvasive way to collect longitudinal imaging data in transgenic mice.
At present, no model mimics all the variables of classic Parkinson’s Disease, which include dopaminergic and nondopaminergic neuron degeneration, central and peripheral nervous system effects, and motor and nonmotor symptoms. Inga Fricke from the Institute for the Advancement of Technology for Health in Toronto, Canada thought transgenic mice expressing firefly luciferase in neuroblasts might offer a novel tool for researchers to observe neurogenesis following artificial induction of Parkinson’s Disease.
Fricke and her team acquired mice genetically altered such that their neurons expressed the luciferase gene and unilaterally injected the mice with 6-hydroxydopamine, a hydroxylated analog of dopamine with neurotoxic properties. The compound was discovered 50 years ago and became notable for its selective degeneration of sympathetic adrenergic nerve terminals. This gave rise to the novel idea of “chemical denervation” in neurobiology, in which a neurotoxin is used to target a specific cell population. When used to lesion nigrostriatal dopaminergic neurons, 6-hydroxydopamine treatment mimics the neuronal damage caused in Parkinson’s Disease.
Fricke and her team imaged the mice using MRI and PET scans at regular intervals after the lesion. They discovered that the blood-brain barrier, the permeable boundary around the brain that permits or denies entry to compounds in the blood stream, has an important role in bioluminescence in neuroblasts arising from post-lesion neurogenesis.
It turns out that the blood brain barrier is compromised by the injection procedure used to lesion the nigrostriatal dopamine cells. This weakening of the barrier permits more luciferin to enter the brain from the blood. Luciferin is the substrate upon which the luciferase acts. As luciferase in transgenic mice is formed only in neuroblasts, Fricke and her team found that signal strength grew as time progressed post-lesion. But the team also noted that the compromised blood brain barrier was responsible for adding to the bioluminescent signal strength and could thus create false positives.
Far from advising against using transgenic mice with the unilateral injection model, Fricke and her team wrote that researchers should simply note the integrity of the blood brain barrier when collecting data. Aside from this caveat, lighting the brain from within seems to be a plausible option to gain a view of the otherwise hidden world of neural damage and reconstruction in Parkinson’s Disease.