Genome-based diets
What if researchers and physicians could personalize our diets—matching our specific dietary needs to our individual genotypes and lifestyle goals?
Dietary guidelines change regularly, updating as researchers accumulate new data on what food combinations best promote human health and longevity. To define the “balanced diet,” researchers need to match nutritional needs for growth, reproduction, and longevity against the effects of an expansive list of nutrients. This goal is lofty enough, yet some scientists, such as Matthew Piper from Monash University in Australia, are looking even beyond that target, aiming to develop strategies for customized nutrition using data from our genes.
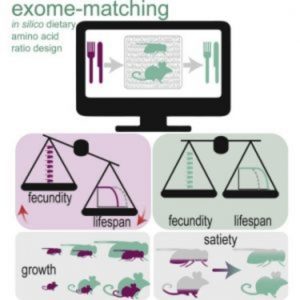
Working with Drosophila in 2009, Piper and his colleagues first discovered that a lack of certain amino acids reduced fertility and that amino acids were critical for determining the fly’s lifespan (1). Since protein levels determine appetite, they decided to follow up on these initial results by looking for the perfect balance of amino acids for fly fitness. They recently reported that a tailored diet, where amino acid quantities and ratios matched the expected requirements as coded in the exome, was more satiating, enhanced growth, and increased reproduction in flies. Beyond fruit flies, they also showed that a diet matched to the mouse exome enhanced growth as well, an indication that the approach might be useful for any organism where a genome sequence is available (2).
“On the practical side, at least for animals that can exist on purified, supplemented diets, it is possible to take a sequenced genome and design the amino acid component to provide them with lower amounts of high-quality protein,” Piper said. “This should enhance long term health while not compromising early life fitness.”
Finding the 20-dimensional Balance
“Understanding how to optimize dietary amino acid content is not trivial as it represents a 20-dimensional balancing problem,” Piper and his co-authors wrote in Cell Metabolism, where they published the results of this study.
To tackle the problem, the team computationally translated all 19,736 predicted protein-coding genes and calculated the proportions of each of the 20 amino acids. They then created FLYAA, a food source where the amino acid ratios matched the demands indicated by the exome sequences.
Of course, some genes are translated at much higher rates than others, so the researchers also took advantage of transcriptome and proteome data to create another dish for the flies: PROTEOMEAA. Surprisingly, the two diets were indistinguishable in the effects they produced on egg laying.
“Measuring the proteome has some technical limitations, and so when analyzing its amino acid content, one doesn’t sample as many sequences as when using the exome-matching approach,” Piper explained. Aside from sampling bias, the team also found that the ovarian transcriptome matched the amino acid ratios predicted by the whole exome more closely than other body tissues. “This could be interpreted as an advantage because it means the amino acids in the egg are found in a proportion that is well suited for making new biomass with minimal waste—a fact that could be of critical importance during embryogenesis in the sealed environment of the egg.”
To find out how flies fared when consuming FLYAA, the team compared them to flies fed a diet established in the literature for supporting laboratory flies, MM1AA. While flies preferred FLYAA when given a choice, they also consumed far less FLYAA than MM1AA when only one food source was available. But flies eating FLYAA produced more eggs and showed increased growth.
When the team compared MM1AA to the exome-matched diet they created, they found that its amino acid ratio differed significantly even though the overall quantity of amino acids was the same. Piper wondered if the effects FLYAA elicited resulted from interactions between the amino acids in the food—for example, different ratios may create different tastes, smells, or textures to alter fly preferences.
To explore this possibility, he and his team created MM2AA, which included the same proportion of exome mismatches as MM1AA, but differed in the relative abundance of the amino acids. They found no difference in fly responses to MM2AA and MM1AA. “The MM2AA control allowed us to build an argument that complex amino acid interactions were not responsible for the effects of amino acids on egg laying that we saw, but instead, the most limiting essential amino acid was key,” Piper said.
The researchers next turned their sights to a mammalian model to test potential for translating the approach. They created an exome-matched diet for mice and found that mice fed accordingly were bigger than their counterparts fed normal mouse chow. They also used less energy to generate heat, showed significant improvements in bone structure, and ate approximately 15% less food.
Lost in Translation
Since both flies and mice consumed less of their respective exome-matched diets and used them more efficiently, it’s possible that exome-matched nutrition may one day prove useful in helping counter a number of food-related problems, such as looming shortages, increasing environmental impacts from farming, and the substantial costs of high-protein diets for livestock and laboratory animals. Piper suspects that efforts towards these goals are already underway in labs around the world.
This early success also raises the possibility of tailoring human diets towards different outcomes, such as building lean body mass or reducing certain types of fat. “I think this is indeed a big step towards achieving this,” Piper said. “Delivering low amounts of high-quality protein could be important for maximizing nutritional efficiency in certain applications, such as for kidney disease or for sports nutrition.”
In these initial studies, Piper and his team focused only on amino acid balance in cases where animals had no choice of what they ate—not a condition that will be reflected outside the lab for animals or humans. And amino acids are not the only nutrient of concern; developing real-world diets will require understanding exactly how other vitamins, minerals, and chemicals required by the body affect health. “Understanding and answering the question, ‘A balanced diet for what outcome?’ is a key step that should be considered,” Piper said. “Finally, understanding how real foods with blends of macronutrients translate to biologically relevant nutrition is a major hurdle to overcome.”
The role of appetite and satiety must also be considered. In this and another study (3), Piper’s team noted that single amino acids and gut microbes modulate appetite, adding even more complexity to the picture.
For now, Piper’s team is focused on understanding why an exome-matched diet influences egg laying in flies, while lifespan is less affected. “We hope that this point of difference in the responses may reveal something about the mechanisms of why protein is such a potent modulator of lifespan,” he said.