Novel nanotube stamp system inserts proteins inside cells
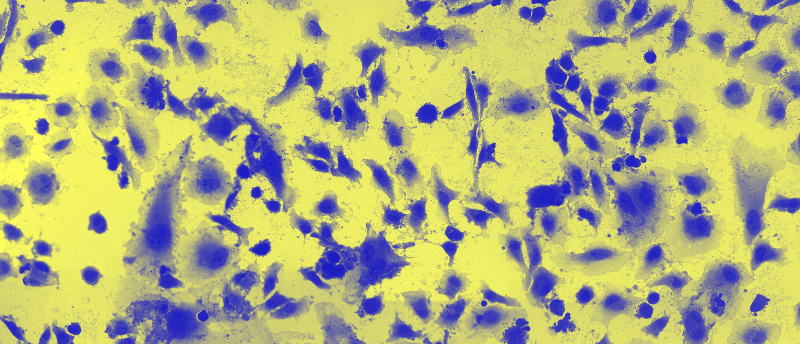
Researchers have developed a stamp coated in nanoscale tubes capable of delivering proteins directly inside cultured cells with minimal damage.
Researchers at Waseda University (Tokyo, Japan) have recently developed a new approach to insert proteins directly into cultured cells. This was achieved using a stamp system covered in hybrid nanotubes (HyNTs), which can mechanically transfect adhesive cultured cells en masse. This technology has potential applications in the direct delivery of cancer therapeutics into cells.
The transfer of genes inside cells is a crucial technique in experimental biology and modern therapeutics. However, methods associated with the delivery of nucleic acids and the subsequent expression of the proteins they encode have certain drawbacks. Issues such as gene silencing and mutation can prevent the successful expression of the desired gene products. This makes the delivery of mature proteins directly into cells an attractive alternative strategy to circumvent these issues. However, as proteins fold into complex 3D structures, direct delivery into cells has its own set of challenges.
In a bid to develop a successful method of protein delivery into cells, the researchers created HyNTs by depositing a polymer called PEDOT onto previously established gold nanotube membranes. These were then assembled on a glass tube to produce the stamp system.
It’s an art: a ‘paintbrush’ precisely delivers nanoparticles to single cells
Researchers have developed a new method for precisely delivering nanoparticles or small molecules to the cell surface, contributing to next-generation therapeutic approaches.
The successful delivery of the fluorescent dye calcein into cultured HeLa cells confirmed that the stamp system was capable of delivering cargo intracellularly to cultured cells. The HyNT stamp system was then used to deliver the enzyme lactate oxidase, labeled with a fluorescent antibody, to both cultured cancerous HeLa cells and healthy mesenchymal stem cells.
Overall, this method was found to be highly effective, with an 89.9% delivery efficiency and post-transfection cell viability of 97.1%. The enzymatic action of lactate oxidase effectively killed the cancerous HeLa cells and not the mesenchymal stem cells. This demonstrates that the HyNT stamp system is capable of delivering proteins that specifically kill cancer cells with minimal harm to healthy cells.
While these results only demonstrate the successful and specific killing of cancer cells in cultures, the researchers hope that this approach could have applications in the clinic. “Our findings highlight the promising efficacy of intracellularly delivered LOx [lactate oxidase] in selectively targeting and killing cancer cells, while sparing healthy cells, offering a targeted therapeutic strategy for cancer treatment,” elaborated Takeyo Miyake, co-corresponding author of this study.
This approach has potential outside of cancer therapeutics, as researchers were also able to insert protein tags capable of binding to intracellular proteins. Subsequent analysis of these tags could reveal a wealth of data regarding the structure and interactions of proteins inside cells.


