Epitranscriptomics and the next wave of cancer therapies
Recent studies revealed critical roles for an abundant RNA modification in cancer biology that might offer promising molecular targets for epitranscriptomic-based therapies.
RNA modifications play a role in glioblastoma (7).
Researchers have found more than 150 unique RNA chemical modifications in different organisms. Although many of these chemical modifications were discovered decades ago, their functions remained largely mysterious until recently. With advances in mass spectrometry and high-throughput sequencing techniques, research into RNA modifications is progressing at an accelerated rate and hints about their functions have begun to emerge (1).
In eukaryotes, the most abundant internal mRNA modification is N6-methyladenosine (m6A)—methylation at the 6 position of adenosine. The identification of proteins that install, recognize, and remove this and other marks revealed roles for mRNA modification in nearly every aspect of the mRNA life cycle, as well as in various cellular, developmental, and disease processes (2).
In 2010, Chuan He at the University of Chicago proposed that RNA modifications such as m6A could be dynamic and reversible, and may significantly impact the regulation of gene expression (3). His team confirmed this hypothesis in 2011 with the discovery of fat-mass and obesity-associated protein (FTO), the first enzyme known to demethylate m6A on RNA, followed two years later by the discovery of a second m6A demethylase called alkB homologue 5 (ALKBH5) (4,5). These revelations rekindled interest in the biological relevance of m6A and sparked extensive research on RNA post-transcriptional modifications in this new era of epitranscriptomics (1).
“My laboratory initiated studies of gene expression regulation affected or controlled by dynamic RNA methylation,” He said. “We realized that this is a mechanism to also control cell differentiation, proliferation and growth, so it was natural to wonder if aberrant RNA methylation could impact human diseases such as cancer.”
Demethylation Drives Blood and Brain Cancers
Several of He’s recent studies revealed the importance of the m6A modification machinery in cancer. As reported in Cancer Cell, they found that FTO is highly expressed in certain subtypes of acute myeloid leukemia, and FTO promoted leukemic oncogene-mediated cell transformation and leukemogenesis (6). The study revealed a previously unrecognized mechanism of gene regulation in tumorigenesis and highlighted the functional importance of FTO and m6A modification in cancer. According to the authors, targeting FTO signaling by selective inhibitors may represent a promising therapeutic strategy to treat leukemia. As FTO has also been implicated in other types of cancers, the discoveries may have a broad impact in cancer biology and cancer therapy.
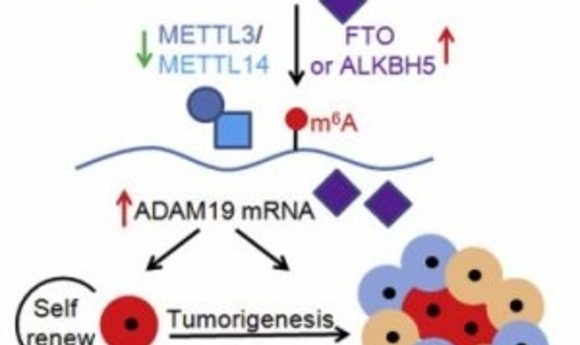
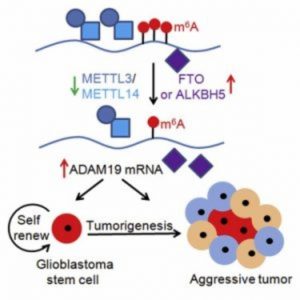
In support of this idea, another recent study by He and his collaborators identified the m6A mRNA modification machinery as a promising therapeutic target for glioblastoma—a malignant brain tumor that develops from astrocytes. As reported in Cell Reports, the researchers demonstrated that m6A mRNA modification is critical for the self-renewal and tumorigenesis of glioblastoma stem cells (7). An FTO inhibitor suppressed the progression of tumors initiated by glioblastoma stem cells and prolonged the lifespan of mice. Moreover, m6A sequencing detected transcriptome-wide mRNA modification and regulation by m6A in glioblastoma stem cells, revealing potential molecular targets for the treatment of glioblastoma.
“The next step in this line of research is to develop more potent FTO inhibitors to target cancer stem cells in the hopes of better suppressing tumor progression and prolonging lifespans, and to determine the mechanisms underlying how m6A RNA methylation regulates the formation of tumors,” said senior study author Yanhong Shi of the Beckman Research Institute of City of Hope. “This work and other studies demonstrate important roles for m6A in regulating multiple types of cancers, such as glioblastoma and leukemia, suggesting that targeting m6A modification machinery can be a general therapeutic tool for a variety of cancers.”
He and his collaborators reported additional evidence supporting a link between m6A and cancer in Cancer Cell (8). The study revealed a role for the m6A demethylase ALKBH5 in glioblastoma, demonstrating that ALKBH5 is highly expressed in glioblastoma stem-like cells. Moreover, overexpression of ALKBH5 was required for the proliferation and tumorigenesis of glioblastoma stem-like cells and predicted poor patient survival. According to the authors, the findings suggest that RNA m6A methylation has a critical role in tumor development and provides a rationale for therapeutically targeting epitranscriptomic modulators in patients with glioblastoma.
“These are the first studies showing that RNA methylation could be directly linked to human cancer,” He said. “It not only opens new directions for basic cancer biology, but also potential new targets for developing therapies.”
Regulating DNA Repair
A major hallmark of cancer is genomic instability, which is associated with the propensity to accumulate DNA damage. As cancer cells often have specific abnormalities in the DNA damage response, chemotherapy and radiotherapy are designed to kill these cells mostly by inducing DNA damage. “It is essential to deeply understand the molecular mechanisms behind DNA repair, as it is of importance for cancer therapy or drug development,” said Benoit Laurent, a postdoctoral fellow in Yang Shi’s lab at Boston Children’s Hospital and Harvard Medical School.
In a study published in Nature, Laurent and Shi, in collaboration with He’s group, reported that RNA m6A methylation regulates the ultraviolet-induced DNA damage response (9). Within two minutes after ultraviolet irradiation, RNA m6A methylation was transiently induced at DNA damage sites. Consistent with the role of DNA polymerases as the downstream effectors of the repair pathways responsible for UV resistance, DNA polymerase κ (Pol κ) localized to damage sites simultaneously with m6A RNA. Taken together, the findings suggest that m6A RNA serves as a beacon for the selective, rapid recruitment of Pol κ to damage sites to facilitate repair and cell survival.
According to Laurent, the study opens a novel and exciting research era in the field of DNA damage and repair. “Scientists involved in research on DNA damage have now to take in consideration RNA as another layer of complexity in all established molecular repair pathways. In addition, they need to consider the importance and the diversity of RNA modifications,” he said. “This last point is particularly interesting, as all the enzymes that deposit or remove modifications on RNA are druggable. It opens a new window for the development of new types of cancer therapy, and could also be of importance for the treatment of cancer recurrence.”





