Cancer culture
Organoid culture is facilitating cancer profiling, screening and treatment. Whether it’s by providing a model to genetically sequence or a platform for drug testing, these 3D structures are an extremely useful tool for cancer research.
Organoids are models of organs produced by culturing cells that recapitulate that organ’s function. They can be grown in vitro from targeted tissues or stem cells that can then organize themselves within a 3D culture. It can be difficult to create organoids because finding the optimal mixture of nutrients, growth factors, cells and techniques for the culture isn’t easy. However, once generated, organoids can provide fantastic personalized laboratory models for experimentation and research.
This article will investigate the use of organoids in cancer research. From genetic sequencing and the recreation of tumor cells to optimizing chemotherapy and drug screening, we will explore the latest developments in organoid research and what it means for treating cancer.
Genetics
Scientists from the Wellcome Sanger Institute (Hinxton, UK) and Hubrecht Institute (Utrecht, Netherlands) have shown that not only is every bowel cancer tumor genetically different, but every cell within that tumor has a unique genetic profile. The research, published in Nature, uses the latest organoid and single-cell techniques to further our understanding of bowel cancer and its mutational processes [1].
Colorectal cancer is responsible for 10% of all cancers. It’s known that each colorectal tumor cell contains subclones with different genetic make ups that makes them respond differently to treatment. However, until now, studying individual tumor cells has been difficult.
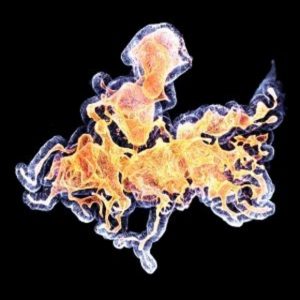
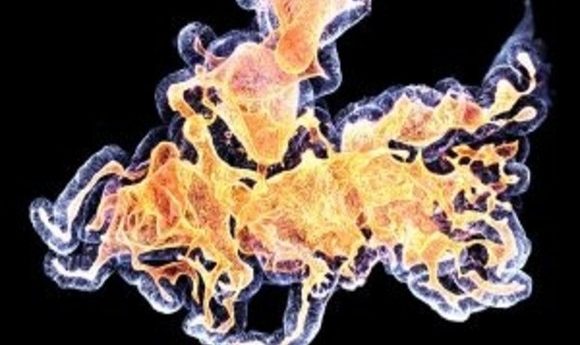
Human colon organoid. Credit: Anne Rios and Florijn Dekkers, Princess Maxima Center
To grow the organoids the team needed, they sampled bowel stem cells and tumor cells from three patients with colorectal cancer. Corresponding author, Hans Clever, explains that doing this from single cells had not been done before, and was a crucial step in their research.
“Organoids had not been used to study single cancer cells before. Nobuo Sasaki in my lab isolated multiple single cells from the tumors and grew them up as organoids. This enabled us to study each cell without the errors that standard single-cell methods bring. For the first time ever, we could make a really comprehensive comparison of individual normal and tumor cells from the exact same type of tissue, taken at the same time, from the same person, and see how the cancer had developed.”
First author of the paper, Sophie Roerink, was surprised by what the group found when looking at the genetics and mutational processes in the cells.
“We found mutational processes in these cancer cells that are just not seen in normal cells, leading to a huge increase in mutation rate for tumors compared with normal cells. This then leads to remarkable genetic diversity within the tumors. We knew before that cancers contained subclones, but this is the first time that anyone has shown that each cell in a tumor is different.”
The team identified that the mutational processes were very different in the cancer cells, and that the mutation rate began to change years before diagnosis. The group proffers that his change in rate could be a useful diagnostic tool in catching the cancer early, as joint corresponding author Mike Staetton explains.
“This study gives us fundamental knowledge on the way cancers arise. By studying the patterns of mutations from individual healthy and tumor cells, we can learn what mutational processes have occurred, and then look to see what has caused them. Extending our knowledge on the origin of these processes could help us discover new risk factors to reduce the incidence of cancer, and could also put us in a better position to create drugs to target cancer-specific mutational processes directly.”
Identifying treatment
So, as we have seen, culturing the organoids is the first difficult step to overcome before experimentation. Many groups across the world are focusing on specific cancers to culture organoids that can be used guide future treatment for the patients they are cultured from.
Researchers at the Columbia University Irving Medical Center and New York–Presbyterian (both NY, USA) have created personalized bladder cancer organoids. The results, published in Cell, show that these organoids mimic many tumor characteristics such as clonal evolution [2].
The team hopes that their organoids can provide a complementary tool for precision medicine practices. Current practice is to identify the specific mutations in a patient’s tumor, but the group hopes their organoids can be used to test potential drug treatments to help determine the patient’s response.
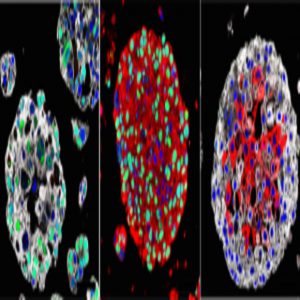
Organoids created from the bladder cancers of patients mimic the characteristics of each patient’s tumor and may be used in the future to identify the best treatment for each patient. Images: Michael Shen / Columbia University Vagelos College of Physicians and Surgeons.
Lead author Michael Shen is excited by the prospects of the organoids.
“The great advantage of organoids is that they are essentially avatars of a patient’s tumor. Having these personalized laboratory models, which we can make in a matter of weeks, will let us test multiple different drugs on the tumor and help us bring precision medicine to individuals with bladder cancer.”
The team sampled cancer cells from 22 patients with bladder cancer. They exhibited many of the properties of their parent tumors, crucially including clonal evolution.
“Clonal evolution is a major driver of tumor progression and drug resistance. It’s remarkably difficult to model for a solid tumor. With these organoids, we will be able to study how bladder tumors evolve and perhaps learn how to prevent tumors from becoming resistant to treatment,” explained Shen.
Personalized treatment is an important next step for bladder cancer. Many animal models do not sufficiently reflect the biology of the cancer and current treatments for bad cases include intensive chemotherapy and surgery. Co-author John Latimer believes that understanding this disease using these superior models of the tumor is the best way to find new treatments.
“The creation of bladder cancer organoids is an important advance in the field. This should greatly improve our understanding of the genomics of bladder cancer, how these tumors respond to drugs, and how they develop drug resistance. Ultimately, this may allow us to develop new therapies for the disease and predict an individual patient’s response to treatment.”
Researchers at the Cold Spring Harbor Laboratory (NY, USA) have also been working on creating 3D organoids to facilitate personalized medicine but for pancreatic cancer. Their study, published in Cancer Discovery, shows that organoids derived from pancreatic cancers can quickly and accurately demonstrate treatment response through organoid profiling [3].
Pancreatic cancer has a poor survival rate with only 8% of patients surviving past 5 years after diagnosis. Currently, the only effective treatment is surgery, but the fast progress of the disease prevents this option for most patients.
The study’s lead, David Tuveson, identified organoids as a solution to this problem. Only a small biopsy is required, and the organoids then take only 6 weeks to grow. He believes that their research shows that organoids can be part of an effective pipeline for drug testing.
“We would culture the organoid from the patient’s cancer and then test all possible standard-of-care drugs as well as experimental drugs,” explains Hervé Tiriac, first author on the paper.
To measure the gene activity of the organoids after chemotherapy, they assessed the RNA levels in the organoids. This process determines the gene signatures that predict sensitivity to treatment. Three of the signatures were able to identify a large number of patients who had shown promising responses to the drugs. Tiriac continues:
“Therefore, the signatures should enable physicians to choose the correct chemotherapy for pancreatic cancer patients for first-line treatment,”
The patients (or their organoids) who did not exhibit the signatures that responded well to treatment could be identified for more urgent care or experimental drugs.
New techniques
The importance of deriving organoids is clear, but doing so is not easy and that is where the development of new techniques can help accelerate their production.
Scientists at the National University of Singapore (Singapore) and a host of other Singapore institutions have specially engineered 3D scaffolds for growing liver tumor organoids. The team hopes their method, published in Biomaterials, has the potential to facilitate high-throughput drug screening [4].
The group identified that current preclinical tumor models were insufficient, resulting in the failing of many potential drugs. Patient-derived xenografts are organoid models that have been developed to more accurately represent the patient’s tumor. However, they are expensive and time consuming to make and until now, growing them in culture had been very difficult.
The team’s new method uses synthetic 3D scaffolds constructed from plant-based hydrogel. This allows the organoids to grow and maintain their structure and function. They examined tumors from 14 liver cancer patients and found that most of them exhibited the properties of their parent tumors. This included intra-tumor heterogeneity; a property of liver cancer tumors in which different populations of cells are present within the tumor.

(From left) Dr Toh Tan Boon, Dr Eliza Fong, Professor Hanry Yu, and Dr Edward Chow with the scaffold of the HCC organoids.
Lead author Eliza Fong is pleased with the outcome.
“This study truly epitomizes the positive synergy we can achieve in growing patient tumors outside the body by marrying advances in tissue engineering with cancer biology.”
The team designed their scaffolds to be 100 microns. This allows them to easily fit within a 96-well microtiter plate, which is a standard platform for high-throughput drug screening. This means that many different drugs can be tested on a sample simultaneously.
Like the previous studies mentioned, co-author, Tan Toh is excited about the promise this technique has for personalized medicines.
“Having a reliable platform to grow liver cancer patient-derived cells is a major step in personalized medicine as we can now use them for increased throughput drug sensitivity testing.”