How Is Type 2 Diabetes and Mad Cow Disease Similar?
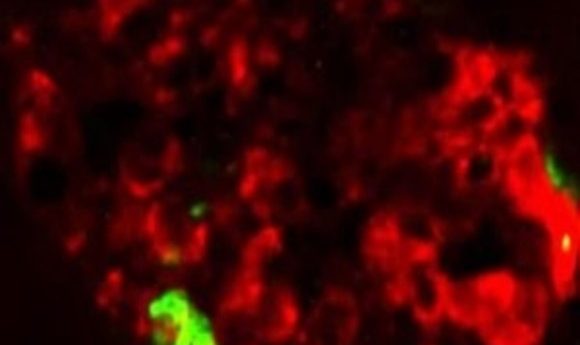
How is type 2 diabetes and mad cow disease similar? A recent study showed that misfolded protein aggregates in pancreatic tissue can act as seeds to spread type 2 diabetes processes, similar to the self-propagation of infectious prions.
Read more on “How Does Diabetes Spread? Mad Cow Disease Could Be the Answer.”
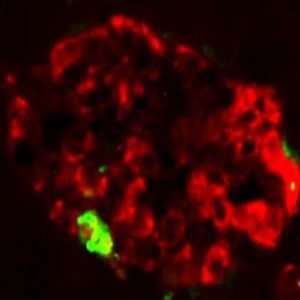
A large protein aggregate (green) forms in a pancreatic islet (red) from a transgenic mouse injected with extract containing misfolded IAPP (1).
Some of the symptoms of type 2 diabetes might be transmissible through a mechanism similar to the one by which prions propagate in mad cow disease, according to a study published in the Journal of Experimental Medicine. The findings suggest that misfolded aggregates composed of islet amyloid polypeptide (IAPP) deposited in pancreatic tissue during the development of type 2 diabetes can act as seeds to spread that process throughout a person’s pancreatic islets or even to other individuals. In this respect, type 2 diabetes could be similar to other diseases associated with misfolded protein aggregates, such as Alzheimer’s disease, Parkinson’s disease, and prion disorders.
“Our data therefore opens up an entirely new area of research with profound implications for public health,” said senior author Claudio Soto of the University of Texas Medical School at Houston. “This prion-like mechanism may play a key role in the spreading of the pathology from cell to cell or islet to islet during the progression of type 2 diabetes.”
Type 2 diabetes is characterized by insufficient insulin secretion from the β-cells of the pancreatic islets, coupled with impaired insulin action in target tissues. More than 90% of patients have islet amyloid deposits, which are composed predominantly of a misfolded and aggregated form of IAPP; however, it has not been entirely clear what role these aggregates play in type 2 diabetes.
Recent studies suggested that the process of protein misfolding and aggregation implicated in various protein misfolding disorders can be transmitted in a way similar to the transmission of prion diseases. In the new study, Soto and his team aimed to provide proof-of-concept for this phenomenon in diabetes.
Their results showed that pancreatic IAPP aggregates can promote the misfolding and aggregation of endogenous IAPP in islet cultures obtained from a transgenic mouse model overexpressing human IAPP or from a healthy human pancreas. In association with IAPP accumulation, animals developed some of the typical clinical abnormalities of type 2 diabetes, including high blood sugar levels, impaired glucose tolerance, and loss of β cells. Taken together, the findings suggest that misfolded IAPP can self-propagate in a manner reminiscent of infectious prions and that IAPP accumulation may have an important role in islet dysfunction in type two diabetes.
However, more research is needed to determine the extent to which the spreading of IAPP aggregation may contribute to diabetic alterations. “Considering the experimental nature of the models and conditions utilized in this study, the results should not be extrapolated to conclude that type 2 diabetes is a transmissible disease in humans without additional studies,” Soto said.


