Low fat CRISPR
CRISPR/Cas9 variant turns off gene associated with high cholesterol
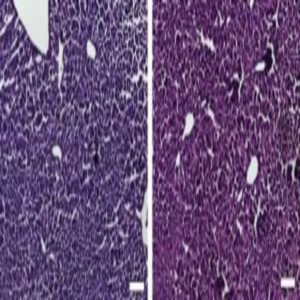
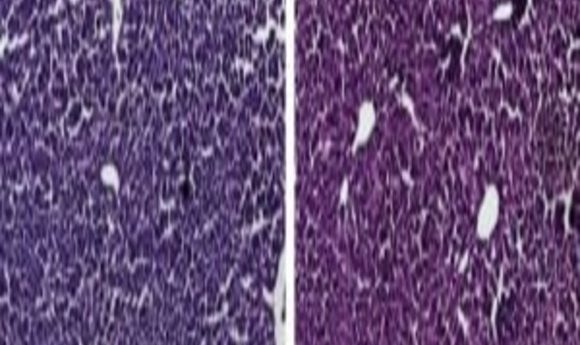
Histological sections of liver from control mice treated with saline (left) and the CRISPR/Cas9 epigenetic repression system in which cholesterol levels were lowered (right) show generally normal and healthy tissue. Credit: Charles Gersbach, Duke University
Researchers from the biomedical engineering department of Duke University (NC, USA) have utilized a CRISPR/Cas9 genetic engineering technique to silence a gene that regulates cholesterol concentrations in murine models. The results of the experiment, published in Nature Communications, are promising for the clinical application of CRISPR as it is the first time this targeted therapy has been successfully delivered in animal models.
The gene that regulates cholesterol levels is Pcsk9. Silencing the PcsK9 gene would have a greater effect on cholesterol than current drugs that target it. Instead of blocking the receptor, the CRISPR therapy would stop it being generated in the first place. The researchers had already seen success in cultured cells but wanted to take the therapy to the next level, as author Charles Gersbach explains.
“We previously used these same types of tools to turn genes on and off in cultured cells and we wanted to see if we could also deliver them to animal models with an approach that is relevant for gene therapy. We wanted to change the genes in a way that would have a therapeutic outcome, and Pcsk9 is a useful proof-of-concept given its role regulating cholesterol levels, which in turn affect health issues like heart disease.”
However, to administer the therapy the researchers had to repackage it and develop a new delivery system. For this they decided to use viral vectors. These viruses are great for targeting specific tissues for gene therapy trials but they only have a small cargo limit. Conventional Cas9 is too large so the team turned to alternatives. Conveniently there is a smaller variant of Cas9 that is derived from Streptococcus aureus, unlike the common variant which is derived from S. pyogenes.
Now the scientists had developed the Cas9 that suited their needs and the delivery system to get it to the target, they also wanted to modify the function. They only needed Cas9 to bind to the target whilst another associated protein, KRAB, did the work of silencing the gene. So, the team deactivated the DNA slicing function creating dead Cas9, aka dCas9.
Despite the success of the therapy in silencing PcsK9, there was evidence of a slight immune response to the treatment. However co-author Pratiksha Thakore thinks that understanding this response is the next step CRISPR therapies must take to translate to the clinic.
“Following injection, we saw that levels of our target gene, Pcsk9, were reduced, but we also observed increases in expression of many immune cell genes, which indicates that immune cells were infiltrating the liver after we delivered Cas9 to the mice. Gaining a better understanding of this immune response and how to modulate it will be important for using Cas9 technologies for therapies.”
It seems the success of clinical treatment using CRISPR enzymes will rely on how the body responds to them. The variety in the bacterial CRISPR family generates many new possibilities but each protein will carry a different risk of immune response. Despite this looming hurdle Thakore is excited by the potential outcomes that can be generated by new experimentation and discovery.
“There are still lots of things for us to explore with this approach. CRISPR/Cas9 tools have worked so well in cell culture models that it’s exciting to apply them more in vivo, especially when we’re examining important therapeutic targets and using delivery vehicles that would be relevant to treating human diseases.”