A postman particle: delivering therapeutics for osteoarthritis
A novel nanoparticle for drug delivery has been developed, which has proved effective in delivering therapeutics for osteoarthritis and preventing cartilage breakdown.11040
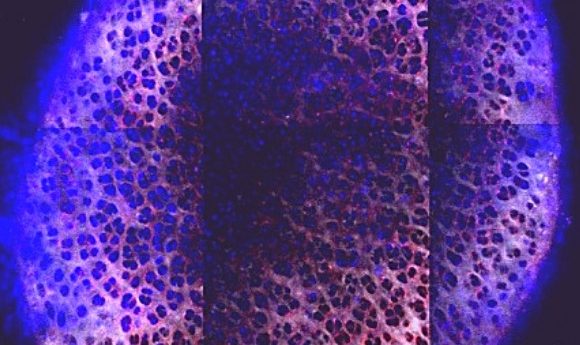
The penetrance of the nanoparticles (blue) through the cartilage, 6 days after administration. Credit: Brett Geiger and Jeff Wyckoff
Researchers from MIT (MA, USA) have developed a new nanoparticle to assist in drug delivery for osteoarthritis therapeutics. The study, led by Paula Hammond, showed clear evidence of enhanced joint protection in rats when compared with therapeutics delivered without the nanoparticle.
Osteoarthritis is characterized by the degradation of cartilage tissue, produced by chondrocytes, and can be due to gradual wearing as a result of age or of traumatic injury. It results in severe joint pain and current therapies focus on alleviating the symptoms rather than treating the cause due to the difficulty in repairing cartilage once it has been damaged and in delivering drugs to the chondrocytes that are affected.
To address the issue of drug delivery, the MIT team decided to design a material that could assist in delivering a drug through the cartilage to the chondrocytes and prevent it from being cleared from the joint before the drug had had a chance to have a therapeutic effect. IGF-1 was selected as the drug intended for delivery as prior investigations in animals had established it as a promoter of cartilage repair and formation.
The researchers were able to develop a spherical molecule with protrusions known as dendrimers emanating from a central body. The dendrimers were positively charged at their ends allowing them to bind to both IGF-1 and the negatively charged cartilage, preventing the drug being washed away. To promote movement through the cartilage the molecule also contained sweeping, hydrophilic PEG branches that briefly displaced the dendrimers’ attachment to the cartilage, allowing the molecule to move through it towards the chondrocytes instead of getting stuck on the surface.
“We found an optimal charge range so that the material can both bind the tissue and unbind for further diffusion, and not be so strong that it just gets stuck at the surface,” explained Brett Geiger (MIT), first author of the study.
Once at the chondrocytes the IGF-1 was then able to bind to receptors on the cell, promoting the formation of proteoglycans, the precursors of cartilage.
When tested in rats, Hammond found that the half-life of the drug and material was four days, 10-times that of the drug alone, and that it remained equal to or above therapeutic concentration for 30 days. What’s more they found that cartilage was significantly less damaged in the joints of rats treated with the particle and drug than the drug alone.
Previous trials that have had success in animals have often gone on to fail in humans. Part of the reason for this is the increase in cartilage thickness from an animal model. In order to address this, the research team showed that the particle developed could successfully diffuse through up to 1mm of cartilage, equivalent to the thickness of human cartilage in a joint.
“That is a very hard thing to do. Drugs typically will get cleared before they are able to move through much of the cartilage,” Geiger says. “When you start to think about translating this technology from studies in rats to larger animals and someday humans, the ability of this technology to succeed depends on its ability to work in thicker cartilage.”
The next step for the research is to test the efficacy of the particle in the delivery of other drugs such as growth hormones, anti-inflammatories and DNA. The success of this particle could mark the start of a change in the approach to treating osteoarthritis, from purely managing the symptoms to tackling the root cause of the disease.