How do flu viruses infect cells? New microscopy method captures complex ‘dance’

How flu viruses enter cells has been directly observed thanks to a new microscopy technique with the potential to revolutionize research on membrane biology, virus–host interactions and drug discovery.
Researchers from Hokkaido University (Sapporo, Japan), Nagoya University (Japan), the University of Zurich (Switzerland) and ETH Zurich (Switzerland) have developed a hybrid imaging system that combines atomic force microscopy (AFM) and confocal microscopy to visualize influenza A viruses infecting living cells live and in high resolution. The fresh insights that this technique provides could lead to the development of much-needed antiviral drugs.
Influenza A viruses are the most common type of flu virus to infect humans, causing epidemics across the globe. Despite their universality, much of how they operate is still shrouded in mystery, particularly when it comes to the very early stages of infection.
We know that the process of viral uptake into cells begins with interactions between proteins hemagglutinin and neuraminidase on the virus’s surface and sialic acid on the cell membrane, which trigger lateral diffusion of the virus and the necessary morphological changes that facilitate endocytosis.
However, observing this in action has proven challenging using conventional microscopy methods. Electron microscopy, for example, can only provide snapshots of influenza A internalization, while AFM is subject to mechanical interference from the cantilever.
With their new platform, the scientists behind the latest study have been able to bypass these hurdles, zooming in on the virus–membrane interaction in unparalleled detail. Called virus-view dual confocal and AFM, the pioneering technology merges aspects of two different microscopy techniques: confocal microscopy and minimally invasive AFM.
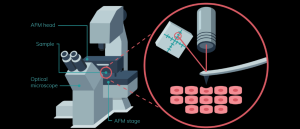 Infographic: Investigating biomechanics with atomic force microscopy
Infographic: Investigating biomechanics with atomic force microscopy
In this infographic, we dive into how atomic force microscopy (AFM) works, the technical features one must consider and how it can be implemented for biomechanical investigation.
The team reduced the disruption caused by traditional AFM by customizing a soft, ‘ultra-narrow’ cantilever with less than half the spring constant of conventional cantilevers. This was used to scan the surface of influenza A viruses and Madin-Darby canine kidney cells, providing morphological images of lateral diffusion and endocytosis. Meanwhile, the confocal microscope generated fluorescence observations.
The result was high-resolution live-cell imaging that captured the dynamics of viral infection directly.
Surprisingly, the team discovered that the cells were not passive in this process; in fact, they appeared to actively promote virus uptake by recruiting clathrin proteins to the virus’s location and bulging to envelop the virus.
“The infection of our body cells is like a dance between virus and cell,” mused lead author Yohei Yamauchi.
By deepening our understanding of how exactly influenza viruses interact with our cells, the findings could herald the development of novel antiviral therapies. The technology could also be applied to study other biological activities at the cell surface membrane.
“Overall, [virus-view dual confocal and AFM] is a versatile tool with the potential to transform future discoveries in membrane biology, virus–host interactions and drug discovery research,” the researchers concluded.