Plankton vs parasites
New research using plankton has revealed that not all hosts are equal when it comes to infectious disease spread between and within species. Understanding this host immunity could be key to preventing future pandemics.
While the world has recently been focused on the zoonotic route taken by SARS-CoV-2 to result in the COVID-19 pandemic, invertebrates are one of the most common vectors involved in transmitting diseases. Overall, vector-borne diseases such as malaria account for almost 20% of infectious diseases globally, resulting in over 700,000 deaths each year and significant economic and public health consequences.
However, epidemiological studies generally assume that an invertebrate host will become infected once exposed to a pathogen – they do not consider that the host itself might have immunity.
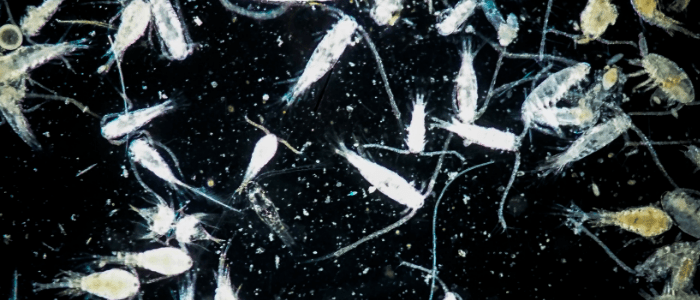
The new research, from Tara Stewart Merrill and colleagues at the University of Colorado Boulder (CO, USA), has demonstrated that is not the case in the tiny plankton species Daphnia dentifera. The team observed that some plankton had the ability to stop the fungal parasite Metschnikowia bicuspidate from entering their bodies, or alternatively to clear it once infected.
Panel discussion: Immunology, from cancer to COVID-19
Our panelists will discuss vaccine development, addressing unknowns in immune response and leveraging the immune system in disease treatment.
“Our results show that there are several defenses that invertebrates can use to reduce the likelihood of infection, and that we really need to understand those immune defenses to understand infection patterns,” explained Stewart Merrill.
If infected, the parasitic spores fill the plankton’s gut and grow until they are released when the host dies. However, over several years of research the team noticed that some of the plankton recovered, with their chance of survival dependent on their internal defense during early infection.
The team went on to use their observations to build a simple probabilistic model for measuring host immunity that can be applied across wildlife systems. The team hopes the model will be useful for predicting future ‘spillover’ events, such as COVID-19.
If used to further understanding for various diseases, the model could lead to a switch in focus from curing to preventing diseases in the first place. One example where this approach could be successful is schistosomiasis, which is caused by a parasitic flatworm transmitted to humans via freshwater snails. Some snails have been seen to have immunological memory.
“We really need to work on understanding prevention of infection, and what that risk is in those aquatic systems, rather than just cures for infection,” Stewart Merrill explained. “If we can better understand how the environment shapes… defenses, we could predict into the future how environmental changes might amplify or suppress risk of transmission to people.”