The birds, the bees and the bacteria: how Wolbachia causes male sterility
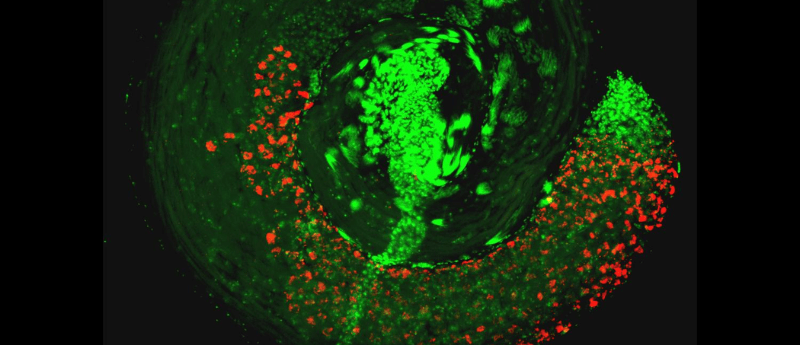
Researchers have uncovered the mechanism underlying male sterility in insects caused by viral proteins present in Wolbachia bacteria.
In a recent study, researchers from Penn State University (PA, USA) elucidated the mechanism by which Wolbachia and the bacteriophage that it carries enables two proteins to block embryo development through their manipulation of long noncoding RNA (lncRNA) and DNA during spermatogenesis. This work could be hugely impactful for the control of insect populations to help combat the spread of diseases such as malaria.
Cytoplasmic incompatibility is a phenomenon whereby bacteria such as Wolbachia, which is found in the reproductive tissue of approximately 50% of insects, render male insects sterile when they mate with uninfected females. This enabled the proportion of the population carrying the gene to increase as uninfected females become less likely to reproduce, while infected females are more successful.
“Wolbachia has genes from a virus called prophage WO integrated into its genome. These genes — cifA and cifB — allow the bacteria to remarkably manipulate sperm and quickly spread through an insect population for their own good,” explained study author Seth Bordenstein.
To find out how the bacteria-virus combination triggered changes in the early stages of sperm development the team undertook experiments focusing on the structure and biochemical function of the Cif proteins. The protein cifA was found to diminish spermatocyte lncRNA, which plays a key role in the transition of histone to protamine, the process that allows chromatin to become highly condensed during spermiogenesis.
 Could the semen microbiome impact male fertility?
Could the semen microbiome impact male fertility?
If you’re starting to wonder whether the microbiome might be to blame for everything, you might just be right.
“By altering this non-coding part of the genome, we found that Cif proteins start impacting sperm right from the earliest stages of development. Wolbachia’s prophage WO genes act like master puppeteers, manipulating sperm development in a way that allows their genes and the symbiotic bacteria to quickly spread through arthropod populations,” Bordenstein added.
This explains the profound effect that Wolbachia has on a critical stage of sperm development, during which the sperm changes from a canoe-shape into a refined needle-like shape which is essential for its ability to travel through reproductive tracts. Highlighting the importance of this process, study author Rupinder Kaur stated that the, “transition is highly conserved in almost everything from insects to humans. Defects in this process can also cause male sterility in humans.”
Furthermore, when the team reduced lncRNA levels they observed more severe cytoplasmic incompatibility. Taken together, both cifA and cifB were observed to be deoxyribonucleases (DNases), causing DNA damage during the later stages of sperm development.
This research has far-reaching implications for managing vectors of human diseases. “One of Wolbachia’s superpowers is that it blocks pathogenic RNA viruses such as Zika, dengue and chikungunya virus, so mosquitos with Wolbachia do not pass these viruses on to people when they bite,” Bordenstein explained.
“So, releases of both male and female mosquitos with Wolbachia in an area where it isn’t already present leads to replacement of the population with mosquitos that can no longer pass on a viral disease. The World Mosquito Program is now using Wolbachia to control viruses in 11 countries,” Bordenstein concluded.
Ongoing pilot studies are already using Wolbachia in this context and also to control agricultural pests. With a better understanding of the mechanisms driving its impacts on fertility, the team hopes that it can optimize the methods used in these studies to improve our ability to control insect-borne diseases.





