Neurons ‘kiss-shrink-run’ to relay information
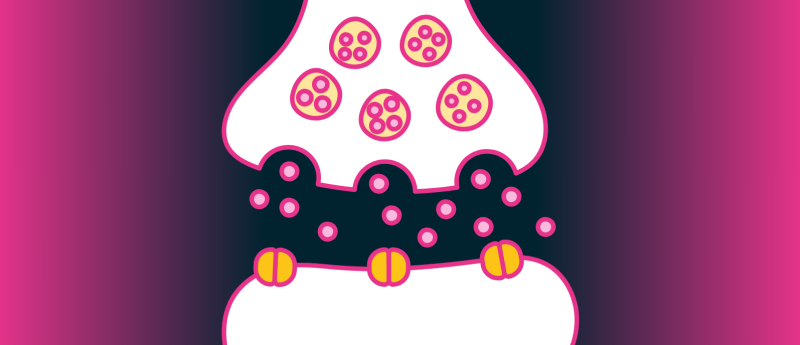
New research has settled the debate between ‘kiss-and-run’ and ‘full-collapse’ fusion to explain how neurons transmit signals across synapses.
Scientists from the University of Science and Technology of China (China) have developed a novel cryo-electron tomography (cryo-ET) technique to reveal the complex process of synaptic vesicle (SV) release and recycling. This unified explanation for synaptic transmission could enable future work on neurotransmission-related diseases such as Parkinson’s or Alzheimer’s.
Synaptic transmission is the basis of neuronal communication. Our brains rely on the efficient and precise relaying of electrical signals, or action potentials, to enable neurotransmitter release. However, there has long been a debate about whether synaptic vesicle release follows a ‘kiss-and-run’ fusion model or an irreversible ‘full-collapse’ fusion model.
In this study, a team led by Guo-Qiang Bi developed a method of time-resolved cryo-ET with high 3D spatial resolution, integrated optogenetic stimulation for synaptic activation and plunge-freezing at millisecond intervals. This technique enabled them to collect over 1000 images of intact synapses in cultured rat hippocampal neurons covering 0–300 ms post-action potential.
 Leptin neural circuit restores balance after anxiety upsets it
Leptin neural circuit restores balance after anxiety upsets it
A brain circuit involving leptin allows mice to carry out vital behaviors, such as eating and sleeping, during anxious episodes.
The images were collected under two different conditions. The first set was captured with a 200 kV Tecnai F20 microscope from Thermo Fisher (MA, USA), equipped with a Gatan (CA, USA) K2 submit camera and direct electron detector. The other set utilized a 300 kV Titan Krios microscope (Thermo Fisher) equipped with either a GIF+K2/K3 camera (Gatan) or a Selectris+Falcon4 camera (Thermo Fisher), along with a post-column energy filter and direct electron detector. The collected images underwent 3D reconstruction with IMOD, an open-source platform developed at the University of Colorado Boulder (CO, USA).
Analysis of these images revealed that at rest, before the arrival of the action potential, SVs were tethered to the presynaptic membrane. Within 4 ms of the action potential, the docked SVs fused with, or ‘kissed’, the membrane, forming a ~4 nm fusion pore before shrinking to pore-opened SVs. Most of these shrunken pore-opened SVs subsequently closed their fusion pores and transitioned into small semi-fused SVs. At 70 ms post-action potential, semi-fused SVs detached, or ‘ran’, from the membrane, ready to be recycled by the body, whilst the pore-opened SVs collapsed.
The team believe that this unified theory could enable better models of neurotransmission as well as provide a general framework for understanding membrane dynamics.