Pumping iron: expanding our understanding of iron-sulfur cluster transport with cryoEM
Research on Atm1 protein’s role in iron-sulfur cluster transport across membranes could improve our understanding of mitochondrial conditions.
A ubiquitous protein family found in almost all eukaryotes, ABC transporters of mitochondria 1 (Atm1) are connected to the regulation of iron accumulation and the formation of iron-sulfur clusters in proteins of the electron transport chain. Proteins containing iron-sulfur clusters play a vital role in the transfer of energy and the creation of adenosine triphosphate (ATP) in mitochondria.
Disruption to Atm1 can cause serious problems; in the human gene ABCB7, a member of the Atm1 family, mutations can cause granular iron accumulation in mitochondria surrounding cell nuclei. This in turn causes ‘sideroblastic anemia’, a condition in which the body produces iron but is unable to integrate it into the hemoglobin. The resulting build-up of iron in the body causes a range of symptoms including fatigue and abnormal heart rhythm, amongst other disorders such as Friedreich’s ataxia.
The ABCB7 protein has been shown to transport molecules, such as iron-sulfur cofactors, across membranes and its importance in iron homeostasis has been demonstrated in previous studies. The molecule glutathione is also known to play a role in this process, binding to iron-sulfur clusters to form a complex that can be transported by Atm1 proteins. The mechanism through which this is accomplished; however, has not been determined.
 Artificial fertilization reveals key protein in sperm–egg fusion
Artificial fertilization reveals key protein in sperm–egg fusion
Researchers discover a fundamental protein in human fertilization, named MAIA after the Greek goddess of motherhood, which could open doors to novel infertility treatments.
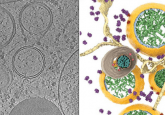
To examine this mechanism, researchers at Ohio State University (OH, USA) identified a very close homolog of ABCB7, found in the fungus Chaetomium thermophilum, named CtAtm1. The team produced large quantities of this protein to study using cryo-electron microscopy and computational modeling.
Using these techniques, they constructed a number of structural models. These models enabled the researchers to detail the pathway utilized by the mitochondria to move the iron-sulfur cofactors across membranes, revealing multiple intermediates of the process and how the glutathione complexed iron-sulfur complexes bound to the Atm1 proteins.
Co-author of the study James Cowan elaborated, “Iron compounds are critical for the proper functioning of cellular biochemistry, and their assembly and transport is a complex process. We have determined how a specific class of iron cofactors is moved from one cellular compartment to another by use of complex molecular machinery, allowing them to be used in multiple steps of cellular chemistry.”
The hope is that this research could contribute to a more complete understanding of disease in humans, of which the team is confident in their contribution, noting that their findings have advanced the understanding of the ABCB7 protein transport mechanism to a significant degree. Cowen expressed excitement at seeing the future developments in medicine and therapeutics from their discovery.
“By understanding how these cofactors are assembled and moved in human cells, we can lay the groundwork for determining how to prevent or alleviate symptoms of certain diseases. We can also use that fundamental knowledge as the foundation for other advances in understanding cellular chemistry.”