Advances in cell engineering therapies: where are we in 2020?
Cell engineering as a field has developed rapidly in recent decades and is now entering therapeutic applications, but which are the key areas to watch in 2020?
Cell engineering may be defined as the substantial manipulation of a live cell so that significant alterations are made to its biological characteristics or physiological functions. With branches in stem cell engineering as well as engineering tissues, the applications are vast and are more and more cell engineering-based treatments are entering the clinical space.
Immune cell engineering: CAR-T therapies
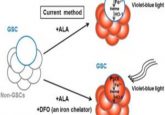
One area generating great interest is CAR-T. Since the first treatments, Kymriah® (Novartis, Basel, Switzerland) and Yescarta® (Gilead Sciences, CA, USA), gained FDA approval in 2017, the treatment, and research into it, has been at the forefront of many an oncologists’ mind.
Whether its integrating CRISPR—Cas9 gene editing to increase the sensitivity of cancer cells to CAR- T cell-mediated death or altering the edited cell’s target molecule, researchers are all looking for ways to improve the specificity of the treatment and make it viable for a wider range of cancer types.
However, despite the current rate of progress, CAR-T is not without hurdles to overcome. Many patients still suffer from unintended side effects due to the toxicity of the treatment and the hostile tumor microenvironment continues to reduce the survival and proliferation of CAR-T cells. Though the reported remission rates of 40-50% demonstrate the potential of CAR-T as a therapy, attention is focused on those in remission, rather than the 50-60% that aren’t. To develop a truly effective therapy, a greater understanding of CAR-T resistance and non-responders is required.
 Talking Tech News | Theo Roth on CRISPR, CAR-T and gene editing ethics
Talking Tech News | Theo Roth on CRISPR, CAR-T and gene editing ethics
BioTechniques talks to Theo Roth from the University of California San Francisco Marson Lab (CA, USA) aboout his groundbreaking CRISPR research, its potential impacts in CAR T-cell therapy and further afield
Tissue engineering
Taking cell engineering to the next level, tissue engineering at the therapeutic level involves combining scaffolds, cells and biologically active molecules in order to create functional and viable living tissues that can restore or replace damaged tissue in vivo.
In current clinical practice, small tissues such as replacement arteries and skin grafts have been created and transplanted into patients, though the procedures are experimental. However, the field holds great potential and, if able to be replicated in humans, lab-based research suggests that much larger organs, such as livers, could be viable for transplantation in the future.
 Tissue engineers get into the groove
Tissue engineers get into the groove
A new method promises to help heal injuries using grooved 3D-printed tissue-engineering scaffolds that can be seeded with living cells.
Stem cell engineering
In regenerative medicine, the stem cell holds vast possibilities for therapeutics and, when combined with cell engineering techniques, the potential of the cells increases. Most recently, researchers from the National University of Singapore have developed a method for returning pluripotent embryonic stem cells to their totipotent state, thereby giving them the ability to differentiate into any cell type, both placental and embryonic.
Normally only found in zygotes, the engineering of totipotent cells opens new avenues for previously unexplored therapies, while also giving researchers greater insight into the earliest stage of embryonic development. The researchers hope that this research will eventually translate into the development of rapid cellular reprogramming strategies with vast clinical applications to benefit human health.
Though stem cell-based therapeutics are becoming more mainstream, the issue of unproven therapies remains. A rise in the number of unregulated private clinics has left many patients vulnerable. Before more stem cell therapies are approved, greater regulation is required so that those most at risk are not left unaware of the safety of a proposed treatment.