Seeking hidden cancer stem cells
Researchers have identified a mechanism that allows cancer stem cells to escape detection. How could this finding pave the way for better diagnostic screening and more effective cancer treatments?
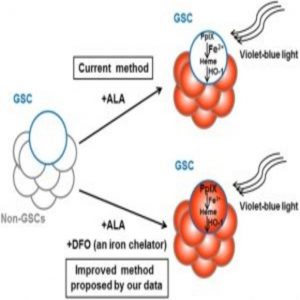
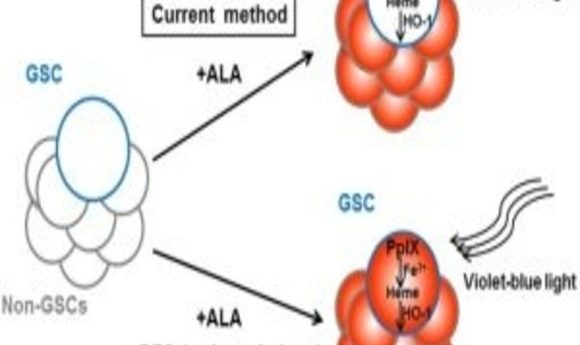
Glioma cancer stem cell (GSC) labelling by the photodynamic agent 5-aminolevulinic acid (5-ALA) is improved by the addition of the iron chelator deferoxamine (DFO).
Credit: Scientific Reports.
Cancer stem cells (CSCs) make up a small fraction of a tumor but are more tumorigenic than other tumor cell types. Capable of producing both CSCs and non-CSCs, these progenitor cells can also differentiate into other tumor cells and are the primary drivers of new tumor growth and cancer recurrence.
Because CSCs are phenotypically distinct from non-CSC tumor cells, researchers at Tokyo Medical and Dental University (TMDU) and Tokyo Institute of Technology were interested in determining whether 5-aminolevulinic acid (ALA), a compound used for photodynamic cancer diagnosis and therapy, could effectively detect rat glioma CSCs. ALA is metabolized to protoporphyrin IX (PpIX), which is both fluorescent and a natural photosensitizer, in tumor cells but not in non-tumor cells. Unfortunately, the clinical use of ALA-based techniques has been hampered by insufficient or non-uniform PpIX fluorescence in the targeted cells.
The researchers treated cultured cells from the rat C6 glioma cell line with ALA and then separated the cells by flow cytometry based on PpIX fluorescence, finding that CSCs were less fluorescent than non-CSC tumor cells. “This is a clear problem for photodynamic detection,” said Tetsuya Taga, corresponding author of a new article in Scientific Reports, in a press release. “The cells that escape detection are the ones that, if not removed, are the most likely to result in treatment failing and a tumor recurring.” To prove this point, the authors demonstrated that the lower-fluorescing CSCs grew into tumors when injected into immune-deficient mouse brains, whereas the higher-fluorescing non-CSC tumor cells did not.
Since PpIX breakdown requires iron, Taga and his colleagues hypothesized that removing iron from the cell culture medium would increase PpIX accumulation and CSC fluorescence. Indeed, when they treated the cells with the FDA-approved iron chelator deferoxamine, “We were able to boost fluorescence accumulation in the highly tumorigenic low-fluorescence glioma stem cell subpopulation,” co-corresponding author Kouichi Tabu said.
Looking for an explanation for the lower levels of PpIX in CSCs, the team found that expression of the heme oxygenase-1 gene (HO-1), a critical component of the PpIX degradation pathway, was upregulated in these cells compared to non-CSC tumor cells and that ALA treatment further increased HO-1 expression. Since HO-1 also is known to protect cells against photodynamic therapy by neutralizing oxidative stress, such as the release of reactive oxygen species by PpIX upon exposure to light, the researchers suggest that this enzyme might be a valuable target for improving both ALA-based CSC detection and cancer drug development.
Overall, the authors have high hopes for the use of iron chelation in combination with ALA to increase detection of CSCs, which could better guide therapy and hopefully reduce the risk of cancer recurrence. “This is particularly exciting because it means this new method has potential to rapidly translate to clinical practice,” said Tabu.