Nanocarriers deliver 2-in-1 cancer treatment

An innovative nanocarrier delivers combination therapy simultaneously, showing promising results in pancreatic cancer mouse models.
Pancreatic cancer remains difficult to treat, partly because it is guarded by biological factors enabling it to resist treatment. Combination therapies have proved to be a useful method for combating stubborn cancers, and in the hopes of optimizing this approach, researchers at the University of California, Los Angeles (CA, USA) have developed a nanoparticle that effectively delivers two different drugs simultaneously.
There has been a significant amount of research investigating ways to assist the immune systems’ defenses to make chemotherapeutics more effective. “In my opinion, invoking the immune system will make a big difference in providing a much better treatment outcome for pancreatic cancer,” said André Nel, the corresponding author of the study. However, combining these two components into one nanocarrier has been challenging. In fact, only one dual-delivery nanocarrier for chemotherapy has been FDA-approved.
The researchers of this study have developed an innovative method to simultaneously package irinotecan, a chemotherapeutic used to treat pancreatic cancer, and 3M-052, an investigational drug that boosts the immune system.
Irinotecan prevents the growth of cancer cells while also sending signals to the immune system’s dendritic cells, which then activate T cells. However, pancreatic cancer often functionally impairs dendritic cells, making the drug less effective. Using 3M-052 in tandem with irinotecan could be a workaround, as 3M-052 can marshal T cells at the cancer site and in nearby lymph nodes, thereby providing extra assistance to destroy the tumor.
Most nanocarriers are made of layers of lipid molecules, similar to cell membranes, with spaces for drug molecules to be packaged. Instead, the researchers of this study created a glass bubble core made of silica, filling the interior with irinotecan and surrounding it by a double layer of lipids. Lijia Luo, the first author of the study, figured out that the 3M-052 molecule’s fatty tail could be used to directly integrate it into the outer lipid layer around the silica bubble.
 Nanoparticle sensors differentiate bacterial and viral pneumonia
Nanoparticle sensors differentiate bacterial and viral pneumonia
Researchers have developed a nanoparticle sensor that can distinguish bacterial pneumonia from viral pneumonia, which could be used to determine the appropriate course of treatment.
This design prevents drug leakage and toxicity while the nanocarrier enters the pancreatic tumor site, where it needs to pass the rope-like barrier protecting the tumor. The silica bubble surrounding irinotecan provides additional protection from leakage, ensuring more of the drug is delivered to the tumor site compared to other nanocarriers.
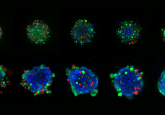
The researchers put their novel dual-delivery nanocarrier to the test in a mouse model of pancreatic cancer and found that it was more effective than both irinotecan without a nanocarrier and the two drugs individually delivered in nanocarriers. It was more effective at shrinking the tumors and preventing metastasis, recruiting more immune cells to the tumor site and maintaining drug levels in the blood for a longer period of time. Additionally, no harmful side effects were observed.
“It traditionally takes 10 to 20 years for new breakthrough technologies to reach the marketplace,” said Nel. “Nanocarriers have been around for almost 20 years. While lipid-based nanocarriers are leading the way, the silica-based carrier decorated with lipid layers stands a good chance of speeding up the rate of discovery and improving cancer immunotherapy.”
Next, the research group needs to carry out more preclinical experiments in large-animal models and assess quality control in large-scale manufacturing of their silica nanocarriers.