Breakthroughs in tumor-seeking bacteria
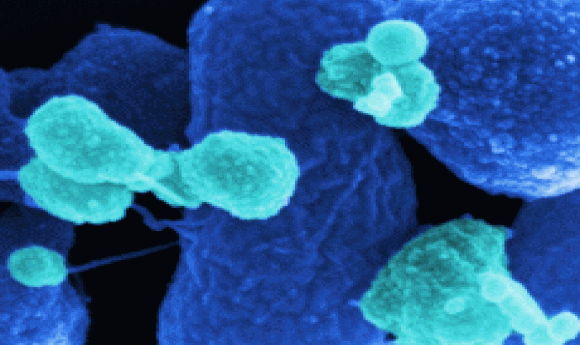
Newly engineered bacterial strains propel an immune-mediated cancer therapy toward clinical applications.
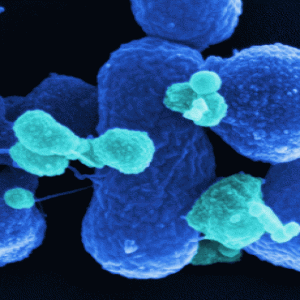
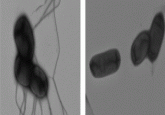
Scanning electron microscopy image of the optimized Salmonella strain SF200 (dark blue). The outer membrane vesicles are stained light blue.
Credit: Manfred Rohde, HZI
In the fight against cancer, researchers have recruited an unlikely ally—a strain of Salmonella that causes food poisoning. Previously, scientists attempting to hijack this pathogen for targeting cancer struggled to balance its aggressive therapeutic actions and harmful attacks on healthy body systems. Now, a team at the Helmholtz Center for Infection Research in Braunschweig, Germany proposed a more precise way to attenuate and strengthen this tumor-fighting technique.
“Understanding the basic immune aspects of bacterial therapies is critical for translating the technology for synthetic biologists like myself,” commented Tal Danino at Columbia University, who wasn’t involved in the Helmholtz studies. “This is great research.”
Hijacking a Versatile Vehicle
When they began their studies, researcher Sebastian Felgner and the Helmholtz team planned to use Samonella as a carrier for vaccines in mouse models, but they soon realized that the bacteria naturally homed to tumors and could attack cancer successfully. Since the team already had suitable strains available, they redirected their focus to tumor-targeting studies.
First, Felgner established some advantages of using bacteria-mediated tumor therapy over conventional methods like surgery or chemotherapy (1). But, “the greatest challenge was, of course, integrating the safety profile of the bacterial strain with therapeutic potency,” said Felgner. “We think we’ve made a tremendous step forward in this matter.”
Using virulent strains of bacteria for systemic cancer treatment can cause severe side effects like weight loss and damage to healthy organs (2). To mitigate these risks by limiting the bacteria’s dissemination throughout the body, the scientists locally applied Salmonella Typhimurium within colon tumor-bearing mice and mice with resistant fibrosarcomas.
This local approach improved safety while retaining the cancer-fighting benefits observed using systemic intravenous applications of bacteria. In fact, the therapy’s accuracy improved enough to allow targeting of secondary or possibly surgically inaccessible tumors as well.
Through genetic engineering, the researchers developed a stronger Salmonella strain, which became their focus in subsequent studies. “Although we incorporated known single mutations, their combination created an unexpected phenotype that significantly increased the efficacy of our strain, SF200,” Felgner explained.
Anticipating Real-World Challenges
Next, the researchers deployed SF200 in mice with antibacterial immunity, anticipating how the anti-tumor bacteria might behave in humans, pets, or livestock that are sensitized to Salmonella(3). They tested the same concept using Escherichia coli, as this close relative of Salmonella is commonly encountered by humans and has an established safety profile.
“Our idea is to attenuate Salmonella and, if necessary, to arm E. coli,” explained Felgner. “This should give us more options for using the bacteria in human cancer patients.”
As expected, pre-exposure to Salmonella or E. coli impaired its therapeutic effectiveness. After comparing systemic and local applications of both bacteria, the team realized that overcoming potential immunity would require further modifications of bacterial-associated immunogenicity factors using genetic manipulation.
“Our Salmonella and E. coli strains activate cytotoxic T-cells involved in tumor clearance,” Felgner noted. “Thus, when we realized the bacteria’s adjuvanticity was exclusively responsible for therapeutic efficacy, we knew it was possible to lower the bacteria’s virulence while strengthening its immunogenicity.”
Looking Ahead
Although the exact mechanism of bacteria-mediated tumor therapy is still unknown, “It appears to be due to a combination of a direct and an adjuvant effect of the bacterial vector,” said Felgner. These findings could prompt other scientists to devise bacterially based strategies against other types of cancer.
Going forward, the researchers will deploy SF200’s unique balance of efficacy and adjuvanticity for upcoming clinical trials in collaboration with Mantis Therapeutics.