Lynch syndrome vaccine could be on the horizon
A cancer-preventative vaccine has shown promise for Lynch syndrome in a preclinical study.
A new mouse study has suggested that a vaccine preventative of cancer could be feasible for patients with Lynch syndrome. The data were presented at the AACR Annual Meeting 2019 (GA, USA; 29 March – 3 April).
Lynch syndrome affects approximately one in 280 individuals in the USA. The syndrome results in an increased risk of cancers of the small intestine, stomach, endometrium, bladder and ovaries, as well as a 70–80% lifetime risk of colorectal cancer. The current main approach to prevention of these cancers in people with Lynch syndrome is frequent screening. Surgery and aspirin can also be used to reduce the risk of colorectal cancer.
The syndrome is inherited and caused by genetic mutations impairing the DNA repair process. This results in mutations in coding microsatellites. While these mutations result in the production of neoantigens, the immune response is not usually sufficient to prevent cancer development.
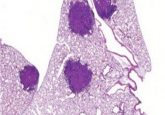
The researchers demonstrated that as few as four neoantigens could be used to safely elicit an immune response, resulting in reduced intestinal tumors and improved survival in a mouse model.
Thirteen coding microsatellites were identified in Lynch syndrome mice that had at least one mutation occurring in 15% or more intestinal tumors. From these, the team determined which mutations generate neoantigens, and their peptide sequences. Vaccination with four neoantigens generated a robust and specific immune response, reducing the median intestinal burden from 61 to 31 mg, and increasing overall survival from 241 to 380 days.
Combination of the vaccine with the NSAID naproxen improved survival to 541 days.
“Our preclinical data are very exciting because they provide strong support for continuing the investigation and development of immunoprevention strategies for patients with Lynch syndrome,” commented Steven M Lipkin, lead author of the study (Weill Cornell Medical College in New York City, NY, USA).