Novel 3D-printed tumor model enables better treatment outcomes
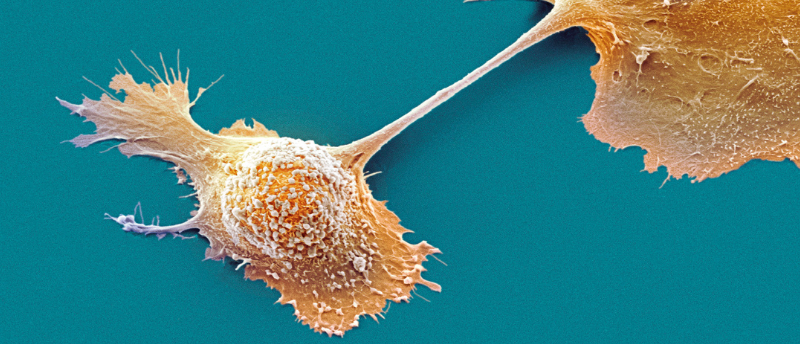
Complex heterogeneous tumors can be better understood with the aid of a new 3-dimensional modeling method.
An international and interdisciplinary team of researchers led by the University of Waterloo (Canada) in collaboration with researchers from Harvard Medical School (MA, USA), Yeditepe University (Istanbul, Turkey) and Brigham and Women’s Hospital (MA, USA) have created a method to produce more accurate 3D models of complex tumors; this tool will allow researchers to study the behavior of cancer cells at a high spatial and temporal resolution.
Conventional 2D models used to study tumors are generated from cells extracted from tumor biopsies that are then grown in a petri dish “For fifty years, this was how biologists understood tumors,” commented Nafiseh Moghimi, the lead author of the study. “But a decade ago, repeated treatment failures in human trials made scientists realize that a 2D model does not capture the real tumor structure inside the body.”
To tackle this issue, the researchers produced a 3D model capable of reproducing key factors of a tumor, including the presence of oxygen and nutrients, chemical gradients and cell-cell interactions. The innovative method combines cutting-edge bioprinting techniques and polymer microfluidic chips. synthetic structures etched with channels that mimic blood flow and the other fluids surrounding a patient’s tumor.
After the microfluidic chips were assembled, the team grew an array of cancer cell types and suspended them in a bioink of their own design. This bioink consisted of gelatine, alginate and other nutrients required to recapitulate the complexity of the tumor microenvironment whilst keeping the cells viable.
 Airway to AirGel: the new in vitro respiratory model
Airway to AirGel: the new in vitro respiratory model
A new organoid model platform has been added to the growing arsenal of tools with which to investigate respiratory conditions.
An extrusion bioprinter was used to integrate layers of different cancer cell types onto the microfluidic chips, mimicking the cellular heterogeneity in a tumor and ultimately producing a living 3D model of complex cancers. This 3D model allows the design of diverse tumor architectures that will more accurately represent how they exist in patients, thus, allowing researchers to study complex cancer cells with high spatial and temporal resolution.
The researchers in this study were especially interested in breast cancer as it is known for the heterogeneity of its tumors. Tumor heterogeneity presents challenges in the treatment of cancers and could be a driver of aggressiveness. As conventional models lack the spatial cellular heterogeneity that is present in breast cancer, a 3D cancer model provides both economic and ethical benefits for predicting how a tumor will respond to treatment. In addition, these models are bridging the gap between 2D models and in vivo studies resulting in fewer animals being sacrificed in pre-clinical trials.
A better understanding of the link between intratumor heterogeneity and tumor response to therapy will bring opportunities for the development of novel drugs and help develop the effective use of approved drugs in new treatment schemes and combinations for more effective personalized therapies.