Airway to AirGel: the new in vitro respiratory model
A new organoid model platform has been added to the growing arsenal of tools with which to investigate respiratory conditions.
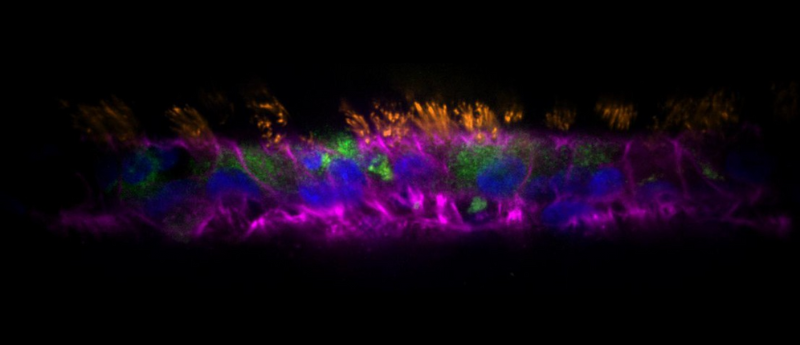
In another breakthrough for the in vitro study of respiratory conditions, a research team from the École Polytechnique Fédérale de Lausanne (EPFL; Switzerland), led by Alexandre Persat, has developed respiratory tract organoids called AirGels. Using these organoids, the team have been able to uncover a mechanism involved in the formation of antibacterial-resistant biofilms during Pseudomonas aeruginosa (P. aeruginosa) infections. This study proves the utility of these new respiratory models and yields a potential target for the disruption of P. aeruginosa biofilms.
Biofilms form when a community of bacteria tightly adheres together and to a surface, often producing a protective matrix that covers the biofilm, shielding it from factors that may try to destroy the bacteria, such as immune cells or antibiotics. Extensive studies of these cultures have been conducted in lab settings but replicating the complex in vivo environment in which they develop is challenging and represents a limitation of these previous studies.
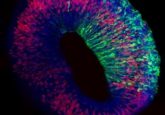
To remove this limitation, PhD student Tamara Rossy and her team took primary human bronchial epithelial cells, sourced from Lonza, and grew them within an extracellular matrix scaffold shaped to mimic the respiratory tract. This approach allowed the model to maintain an intact air–liquid interface in the lumen of the developing organoid, which promoted physiologically accurate differentiation of the primary cells and yielded organoids with a cellular composition that closely mimicked that of the epithelia of the distal human airway.
These models also demonstrated a ciliary beating frequency and directional flow clearance velocity comparable to in vivo physiological measurements. The cultures were maintained on a microfluidic chip that also facilitated luminal access and helped to enable imaging.
Commenting on the complexity and utility of these models, Persat noted that they were, “a game changer,” and that, “there is a lot to say about this study, but the engineering of organoids for infection research has tremendous potential.”
 A breath of fresh air for respiratory disease studies
A breath of fresh air for respiratory disease studies
A novel 3D-cell culture method enables the rapid production of genetically identical ‘lung buds’ for the investigation of respiratory diseases, providing new insights into the pathogenesis of COVID-19.
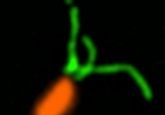
Putting the AirGels to the test, the team infected them with P. aeruginosa and used a combination of high–resolution fluorescence microscopy tools to observe the infection dynamics and biofilm biogenesis at a single-cell level. The microscopes used included a Nikon TiE epifluorescence microscope equipped with a Hamamatsu ORCA Flash 4 camera for low-magnification imaging, a Zeiss Lightsheet Z1 dual-sided selective plane illumination microscope for full-channel cross-sectional imaging and a Nikon Eclipse Ti2–E inverted microscope coupled with a Yokogawa CSU W2 confocal spinning disk unit and equipped with a Prime 95B sCMOS camera for all other imaging.
These observations, coupled with follow-up tests using mutants devoid of flagella and type IV pili, allowed the team to conclude that P. aeruginosa employs retractile filaments, type IV pili, to produce the force required to retract the mucus in the airway and allow the bacteria to aggregate and form a biofilm. This yields a potential target for future treatments to disrupt or prevent the formation of P. aeruginosa biofilms.
Ultimately, this study provides a clear proof of principle for the use of AirGels, adding to the growing list of in vitro models, such as the recent development of lung buds, that can be used for studying respiratory conditions.