Works like a CHARM: the AI model that genomically profiles gliomas
AI can now genomically profile brain tumors during surgery, offering real-time guidance on how best to remove or treat glioma.
A research initiative led by Harvard Medical School (MA, USA) has overcome the challenges of the current intraoperative diagnostic approach for brain tumors by using AI. The new system is called CHARM, which stands for Cryosection Histopathology Assessment and Review Machine; although the tool requires clinical validation and FDA approval, it has shown promise when identifying molecular markers of gliomas, the most aggressive and common form of brain cancer.
Currently, the best options for understanding the molecular makeup of a tumor during surgery is to remove a tissue sample, freeze it and then study it beneath a microscope. This is not an ideal solution as freezing can cause cellular disfiguration, leading to incorrect clinical evaluation. Additionally, the human eye can’t reliably notice genomic variations in tissue, even with high-power microscopes.
CHARM offers an alternative that overcomes these challenges, allowing surgeons to make decisions about a patient’s optimal treatment plan mid-surgery. This would potentially save the patient from having to undergo another invasive, expensive surgery.
 Personalized brain models reveal the smarter the brain the slower the decision making
Personalized brain models reveal the smarter the brain the slower the decision making
Study reveals how brain structure affects intelligence and decision making via in silico modeling of individual brains.
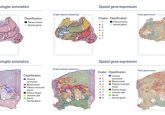
CHARM was trained using 2,334 brain tumor samples from 1,524 people with glioma from three different patient populations. When faced with unseen brain samples, CHARM was able to distinguish molecular mutations of tumors at 93% accuracy. It was also able to successfully classify the three major variations of gliomas based on their specific molecular markers, a feature that informs treatment options and prognoses.
Additionally, the tool was able to identify the visual characteristics of the tissue around the glioma, recognizing signs of more aggressive brain tumors, such as cell death and higher cell density. CHARM’s ability to recognize molecular changes and how these connect to the actual appearance of the cells is important as it provides context that renders the AI model more accurate.
Despite gliomas’ molecular complexity and tumor cell variation, CHARM has managed to successfully profile them in tissue; however, this AI technology will need to be retrained to maintain its accuracy. Additionally, the researchers are sure that, although trained on gliomas, CHARM could be applied to other brain tumor subtypes if retrained.
“The CHARM tool would have to be retrained periodically to reflect new disease classifications as they emerge from new knowledge,” remarked senior author Kun-Hsing Yu. “Just like human clinicians who must engage in ongoing education and training, AI tools must keep up with the latest knowledge to remain at peak performance.”