Novel organoid platform could revolutionize cancer treatment screening
Tumor organoid developed using patients’ cells could pave the way for effective immunotherapy screening, allowing patients access to personalized treatments.
A patient-specific organoid model developed by researchers at the Wake Forest Institute for Regenerative Medicine (WFIRM; NC, USA) has the potential to predict whether cancer patients will respond to immunotherapy. The work, published in Annals of Surgical Oncology, could change the way cancer treatments are screened, meaning patients are able to quickly access the most effective therapy for them.
Organoids are 3D tissue cultures that act as miniaturized versions of tissues and organs. Organoid models of most major organs have successfully been developed, including the pancreas, liver and heart. Organoids can be utilized to model diseases in the hope of identifying causation or treatments, to predict efficacy or toxicity in drug screening and to enhance the knowledge of cellular interaction.
Interested in this piece? Click here to check out the rest of our spotlight on 3D cell culture.
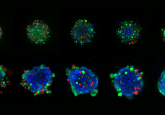
In this latest development, the organoids were created using tumor and lymph node samples from eight stage III and IV melanoma patients. On average, 75–100 organoids were created from each patient’s samples, with a successful establishment rate of 90%, although organoid yield was dependent on specimen size.
Each organoid set was divided into two groups, one containing patient tumor cells only and the other containing additional immune cells obtained from lymph nodes, which were described as immune-enhanced patient tumor organoids.
 New lung model gives lab animals a breather
New lung model gives lab animals a breather
A first-of-its-kind 3D lung model could replace animals in inhalation toxicity research.
Seven days after initiation, both sets underwent screening with the following immunotherapeutic agents: nivolumab, pembrolizumab, ipilimumab and dabrafenib/trametinib. Incubation was maintained for 72 hours.
Immune-enhanced organoids demonstrated a clinical response to immunotherapy in 85% of patient samples. No response was observed in tumor only organoids.
“This bioengineered patient-specific tumor model opens the door to speedier drug screening to get the best therapy to patients as soon as possible and rationalize the use of immunotherapy drugs to the patients that will show a clinical response,” explained study senior author Aleks Skardal (now of Ohio State University, OH, USA)
In collaboration with the Wake Forest Comprehensive Cancer Center (NC, USA), WFIRM has founded the Wake Forest Organoid Research Center (NC, USA), an institute that will aid the collaboration of researchers and clinicians with the aim of developing organoid technologies that improve patient treatment.
Immunotherapy is a promising treatment option for many cancer types; however, it can be costly and response rates vary. The organoids developed in this study could pave the way for personalized immunotherapy screening, potentially saving billions of dollars in wasted treatment and ensuring patients’ time is not wasted on ineffective options.