The protein needed for fertilization
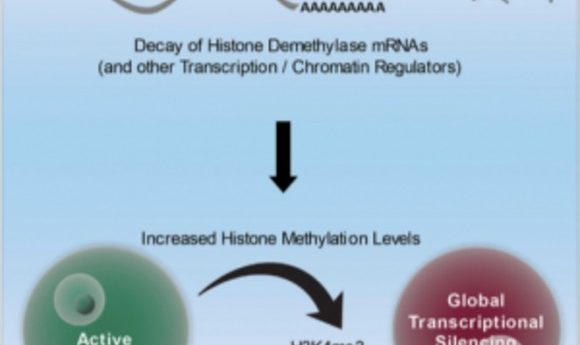
Researchers have uncovered a developmental switch needed to activate global transcriptional silencing, a key step needed for the fertilization of eggs.
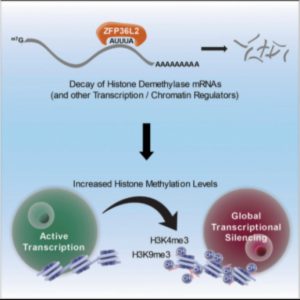
Zfp36L2 acts as a switch toward the end of oocyte development that turns off demethylation and drives the increase in methylation associated with global transcriptional silencing, a process needed to make an egg capable of fertilization (1).
Many couples struggle with infertility; in 15% of cases, the cause is unknown. “Right now in the in vitro fertilization lab we can look at eggs under the microscope and we can say they look normal or they’re a little fragmented and granular, but we have a long way to go to really understand at the molecular level what determines whether or not the quality is there to successfully make a baby,” said Heidi Cook-Andersen from the University of California, San Diego. Cook-Andersen’s team recently uncovered a key protein needed to make eggs capable of fertilization (1).
The study began when Cook-Andersen, came across a paper published in 2004 describing how Perry Blackshear’s group from the National Institute of Environmental Health Sciences knocked out zinc finger protein 36 L2 (Zfp36L2) in mice to study blood cell development (2). The group didn’t get a full knockout, but a partial deletion that caused a complete arrest of embryo development at the two cell stage.
“I got really excited about this because the oocyte-to-embryo transition happens without transcription,” said Cook-Andersen. Transcription is turned off right before ovulation, and it doesn’t come on again until the late two cell stage in mice. Global transcription silencing is key to the oocyte-to-embryo transition, although no one knows exactly why or how the process occurs. This means that gene expression during the oocyte-to-embryo transition is heavily dependent on post-transcriptional mechanisms, such as mRNA decay.
Zfp36l2 activates mRNA decay—a fast mechanism to quickly turn off or on gene transcription post-transcriptionally. “[Blackshear’s] embryos arrested at that two cell stage, just when you would expect the massive mRNA decay event that we think is important for embryo development to happen,” said Cook-Andersen. She contacted Blackshear and a collaboration to study Zfp36L2 was born.
Cook-Andersen hypothesized that mRNA decay by Zfp36l2 expression specifically in the oocyte would be critical for making a developmentally competent egg. The team generated a mouse that lacked Zfp36L2 expression only in the oocytes. Over six months, wildtype mouse mating pairs produced 60 pups, while oocyte-specific Zfp36L2 deficient female mice mated with wildtype males produced none.
Based on Blackshear’s earlier findings, Cook-Andersen thought that when they knocked out Zfp36l2 specifically in oocytes, they would find a defect in the transcriptionally silent embryo. Instead, what they found through histological and transcriptional analysis was an even earlier defect—Zfp36l2 was required for the eggs to undergo transcriptional silencing itself.
Using single cell RNA sequencing, the researchers found that Zfp36L2 binds to and degrades a large group of mRNAs that code for histone demethylases. In the absence of Zfp36L2, oocytes fail to accumulate the histone methylation marks closely associated with global transcriptional silencing. Thus, they are kept transcriptionally active and resistant to fertilization.
Although her team hasn’t looked into human oocytes yet, Cook-Andersen suspects that Zfp36L2 plays a similar role in humans because the pathway is highly conserved and Zfp36L2 decays the same set of histone demethylase mRNAs in human cells. If so, it is possible that abnormal expression of Zfp36l2 might play a role in some currently unexplained causes of female infertility. “We would love to look in humans, but that’s a little bit difficult with limited material and ethical concerns,” said Cook-Andersen.
For now, she is most excited about the basic research questions behind the oocyte-to-embryo transition that remain unanswered. “A really exciting question raised by our findings is how transcription is turned on again after fertilization if Zfp36L2 is degrading things critical for transcription on a global scale in the oocyte. If you get rid of things that are needed for transcription how do you turn it back on without making those things again? Or do you?” said Cook-Andersen.