Why 20 amino acids?
Biochemists shed light on the evolution of our 20- amino acid toolbox.
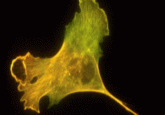
The genetic code of life depends mainly on 20 amino acids, which can be arranged in various combinations to form proteins. For almost a century, biochemists have puzzled over why exactly this number of amino acids evolved, especially since around 13 amino acids would have been sufficient to form functional proteins. Why did the remaining 7 appear?
Led by Matthias Granold and Bernd Moosmann at Johannes Gutenberg University Mainz, a team of researchers used quantum chemistry techniques to analyze the reactivities of amino acids. They identified ways that the chemical properties of these 7 amino acids may have conferred advantages to early life, as oxygen appeared on Earth and provided new evolutionary pressures.
A biochemist studying redox reactions and their appearance in disease, Moosmann made a striking observation several years ago. He found that the amino acids that tended to be oxidized during cell death were also those that appeared latest in evolution.
“After having noticed this pattern—that the typical redox amino acids, and those that are easily destroyed in models of disease, arrived late in the genetic code—we wondered about whether this was a serendipitous observation, or whether there was any true relationship to fundamental properties of the amino acids,” said Moosmann.
To test how reactivity related to time of appearance during evolution, Moosmann and his team analyzed the quantum chemistry of all 20 amino acids, as well as several abiotic amino acids found in meteorites. Based on an analysis of molecular orbitals, they showed that amino acids that arrived later during evolution more readily undergo oxidation reactions.
The researchers hypothesized that the newer amino acids, such as methionine, tryptophan, and selenocysteine, were favored on early Earth because of their reactivity with oxygen. They proposed that these amino acids would have protected early life from oxidative damage. To support this hypothesis, they showed that in culture, cells are less vulnerable to oxidative stress when supplied with lipophilized tryptophan or tyrosine.
Moosmann plans to conduct more biochemical tests to understand how the more recently evolved amino acids affect protein function, especially in terms of redox reactions. “We could replace some of the new amino acids with artificial ones which are not active, but have the same shape and fold the same way in proteins, and see whether there is a specific or general difference in the proteome,” he proposed.