Business of BioTechniques

The past few months have seen a number of big industry collaborations across pharma, biotech, and life sciences, alongside interesting developments that could lead to new and improved tools for genetic engineering. In the drug discovery space, progress has been made towards novel treatment approaches for cystic fibrosis and cancers. At the same time, charities and business accelerators are fostering networking and exchange between all these exciting areas. Here, we present a roundup of the last few months in the business of biotechniques.
Industry Developments
PhoreMost and Boehringer Ingelheim enter multi-project drug discovery collaboration
 PhoreMost, a UK-based biopharmaceutical company dedicated to drugging ‘undruggable’ disease targets, has entered into a multi-project drug discovery collaboration with Boehringer Ingelheim. Under the terms of the agreement, PhoreMost will receive an upfront payment and research funding together with downstream success-based milestones.
PhoreMost, a UK-based biopharmaceutical company dedicated to drugging ‘undruggable’ disease targets, has entered into a multi-project drug discovery collaboration with Boehringer Ingelheim. Under the terms of the agreement, PhoreMost will receive an upfront payment and research funding together with downstream success-based milestones.
During the collaboration, PhoreMost will deploy its in-house expertise and phenotypic screening platform, SITESEEKER®, towards disease relevant pathways nominated by Boehringer Ingelheim. Novel targets identified will be further validated and characterized by Boehringer Ingelheim as part of its internal Discovery Research pipeline. Boehringer Ingelheim’s research program is active in the fields of immunology and respiratory diseases, cardiometabolic diseases, oncology research and immuno-oncology, as well as disorders of the central nervous system.
Owlstone Medical to join EU-Funded EPHOR Consortium to advance occupational health science in order to reduce disease burden from workplace exposure

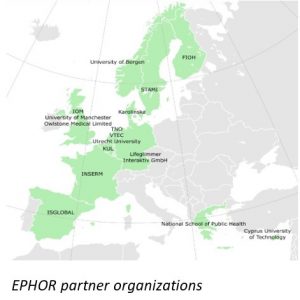
Owlstone Medical, the global leader in Breath Biopsy® for early disease detection and precision medicine, joined the EU-funded Exposome Project for Health and Occupational Research (EPHOR) Consortium.
The consortium aims to further elucidate the complex relationships between environment and disease by developing the concept of the ‘working-life exposome’. This takes into account the influence on health of all occupational and related non-occupational exposures (e.g. lifestyle) throughout the lifespan of an individual. Owlstone Medical will receive funding from EPHOR to provide Breath Biopsy Collection Stations and Kits for the collection of breath samples and will perform analysis on samples received.
Horizon Discovery to provide access to novel base editing technology

Horizon, a global leader in the application of gene editing and gene modulation technologies, announced it will provide access to a novel base editing technology licensed from  Rutgers, The State University of New Jersey, for exclusive use in therapeutic, diagnostic and services applications. This technology is incorporated into Horizon’s next-generation gene editing platform to enable the development of novel therapeutics that rely on engineering patient’s cells either directly in the body (gene therapy), or externally before transplanting back into the patient (cell therapy). This platform will also expand the Company’s research tools and service provisions.
Rutgers, The State University of New Jersey, for exclusive use in therapeutic, diagnostic and services applications. This technology is incorporated into Horizon’s next-generation gene editing platform to enable the development of novel therapeutics that rely on engineering patient’s cells either directly in the body (gene therapy), or externally before transplanting back into the patient (cell therapy). This platform will also expand the Company’s research tools and service provisions.
Base editing is a novel technology for engineering DNA in cells, which has the potential to correct certain errors or mutations in the DNA, or inactivate disease-causing genes. Compared with currently available gene editing methodologies such as conventional CRISPR/Cas9, which creates “cuts” in the gene that can lead to adverse or negative effects, this new technology allows for accurate gene editing while reducing unintended genomic changes that could lead to deleterious effects in patients.
Avacta’s proprietary TMAC cancer therapy platform demonstrates successful proof-of-concept in pre-clinical study

Avacta, the developer of Affimer® biotherapeutics and reagents, demonstrated initial proof-of-concept for its proprietary new class of drug conjugate, tumor microenvironment activated drug conjugates (TMAC™), in a pre-clinical animal model of cancer. The study showed that AVA04-VbP outperformed Bavencio (avelumab), a marketed anti-PD-L1 immunotherapy.
AVA04-VbP combines an Affimer PD-L1 checkpoint inhibitor with an I-DASH chemotherapy warhead, addressing the acute systemic toxicity associated with I-DASH inhibitors by targeting the release of the drug warhead in the tumor microenvironment.
ERS Genomics licenses CRISPR gene editing technology to Daiichi Sankyo to support internal research and development

ERS Genomics provides broad access to the foundational CRISPR/Cas9 intellectual property co-owned by Emmanuelle Charpentier, and has recently signed a license agreement with Daiichi Sankyo, a global pharmaceutical company with corporate origins in Japan.
Under the terms of the license, Daiichi Sankyo is able to use CRISPR technology to support its R&D initiatives to address areas of unmet medical need.
Evonetix collaborates with imec to scale-up chip-based technology production for third generation DNA synthesis platform

Evonetix, the synthetic biology company developing a desktop platform  for high-fidelity and rapid gene synthesis, has partnered with the research and innovation hub, imec, a research and innovation hub active in the fields of nanoelectronics and digital technologies. This collaboration aims to increase production of Evonetix’s proprietary microelectromechanical systems (MEMS)-based silicon chips, enabling the platform to be manufactured at a commercial scale.
for high-fidelity and rapid gene synthesis, has partnered with the research and innovation hub, imec, a research and innovation hub active in the fields of nanoelectronics and digital technologies. This collaboration aims to increase production of Evonetix’s proprietary microelectromechanical systems (MEMS)-based silicon chips, enabling the platform to be manufactured at a commercial scale.
Evonetix’s technology utilizes a silicon chip, made by MEMS processing, that controls the synthesis of DNA at many thousands of independently controlled reaction sites or ‘pixels’ on the chip surface in a highly parallel fashion. Following synthesis, strands are assembled on-chip into double-stranded DNA in a process that identifies and removes errors, enabling accuracy, scale and speed that is several orders of magnitude better than conventional approaches.
Enterprise Therapeutics publishes paper on novel therapeutic approach for treatment of all cystic fibrosis patients

Enterprise Therapeutics, a biopharmaceutical company dedicated to the discovery and development of therapies against respiratory disease, has published its first peer-reviewed paper in the American Journal of Respiratory and Critical Care Medicine.
The paper describes TMEM16A potentiation via ETX001 as a novel approach for the treatment of cystic fibrosis (CF). The research was conducted in collaboration with the University of Sussex, University of North Carolina and University of Miami.
TMEM16A potentiation offers an approach for the treatment of CF, that may be applicable to all patients including the ≥10% of patients with a combination of nonsense and other rare mutations that aren’t treatable via cystic fibrosis transmembrane conductance regulator (CFTR) modulation. The TMEM16A potentiator can be delivered as a monotherapy or in combination with other therapies such as CFTR repair.
1 – Danahay H L, Lilley S, and Fox R et al., (2020). TMEM16A Potentiation: A Novel Therapeutic Approach for the Treatment of Cystic Fibrosis. American Journal of Respiratory and Critical Care Medicine.
Available at: org/10.1164/rccm.201908-1641OC
IONTAS and Adaptate Biotherapeutics collaborate to identify optimized antibodies for novel cancer targets

IONTAS, a leader in the discovery and optimization of fully human antibodies, entered into a collaboration agreement with Adaptate Biotherapeutics, developers of antibody-based therapeutics for modulation of gamma delta T-cells, which spun-out from GammaDelta Therapeutics in 2019.
The agreement is to generate and optimize antibodies for novel immuno-oncology targets, including access to IONTAS’ proprietary Mammalian Display technology to select antibodies with optimal biophysical properties.
Horizon Discovery signs collaboration and license agreement with Mammoth Biosciences

Horizon has signed a collaboration and license agreement with Mammoth Biosciences (“Mammoth”), to access Mammoth’s novel CRISPR platform and thereby facilitate delivery of a new generation of genetically engineered Chinese hamster ovary (CHO) cells for the production of biotherapeutics such as therapeutic antibodies.
Under the terms of the agreement, the companies will collaborate to optimize certain CRISPR tools to rapidly develop proprietary CHO cell lines. In these cell lines, selected genes are ‘knocked out’ to improve performance parameters such as increasing productivity or reducing naturally-occurring contaminants to facilitate purification.
ELRIG UK and the British Pharmacological Society collaborate to improve scientific exchange in UK drug discovery community

Two major learned societies: the European Research & Innovation Group (ELRIG) UK and the British Pharmacological Society have formed a strategic alliance. The 2-year agreement offers the organizations the opportunity to benefit from each other’s scientific communities to help strengthen their positions and reach. ELRIG and the British Pharmacological Society members will gain access to new openings for scientific exchange and collaboration between industry, academia and the clinic to help drive the successful discovery and development of new medicines across the UK.
Start Codon and One Nucleus partner to support UK life science ecosystem
Start Codon, a new model of life science and healthcare business accelerator, has partnered with One Nucleus, a membership organization for life science and healthcare companies. Under the terms of the partnership agreement, One Nucleus will support and profile the life science and healthcare start-ups participating in the Start Codon accelerator program.
Grants and Funding Rounds
| Recipients | Lead Investors | Funding type | Amount | More info |
| LUNAC Therapeutics | Epidarex Capital
University of Leeds |
Series A | £2.65 million | LUNAC Therapeutics spun out to develop next generation anticoagulants, and announces series A funding round |
| LUNAC Therapeutics
Medicines Discovery Catapult University of Leeds |
Innovate UK | Seed | £3.14 million | LUNAC Therapeutics led project awarded £3.14 million under Innovate UK Biomedical Catalyst program, to develop next-generation anticoagulant |
| Epidarex Exeed | Epidarex Capital | Seed | Not disclosed | Epidarex Exeed founded by Epidarex Capital to advance early stage, highly innovative life science assets |
| Turbine | Delin Ventures | Seed | €3 million | Turbine secures €3 million EUR seed fund to expand the potential of simulation-first drug discovery |
| Mogrify | SBRI Healthcare | Phase II | $1.1 million | Mogrify awarded $1.1M additional funding from SBRI Healthcare |
People
| Organization | Appointments and Information |
| Turbine | 
|
| Avacta |
Paul Fry, Non-Executive Director |
| H.E.L Group | 
H.E.L Group bolsters production and service support for customers in laboratories worldwide |
 Author details
Author details
Zyme Communications provides PR and marketing services for the life science sector to help companies raise their profile and generate interest from commercial leads, investors and partners. With an international network of life science trade media contacts and a strong technical understanding, Zyme is committed to providing real value from objective-led communications. To find out more, visit our website, or follow us on LinkedIn and Twitter.






