Refocusing our microbiome lab for SARS-CoV-2 research
“Does anyone have the ability to pop down to [the hospital] to collect samples from doctors swabbing rooms prior to COVID-19 patient occupancy?” When this email came across the lab listserv from my boss on 10 March 2020, I thought “Sure, I can swing by.” I had been following the news and feeling the impending weight of what was coming, and this seemed like a small productive way to contribute. I went to the hospital, along with one of our postdoctoral researchers and a clinical research fellow, and met with the doctor who had contacted us. I didn’t know that within the next 24 hours, I would drop everything else and completely dedicate my time to leading this new project.
Due to our lab’s expertise in microbiology of the built environment and previous work studying hospital microbiomes [1], a local intensive care unit doctor contacted us with an idea for an urgent experiment. Although the hospital was preparing for the imminent arrival of patients with COVID-19, they were doing so with a global lack of data about this emerging infectious disease. The doctor who contacted us wanted to swab hospital room surfaces before, during and after patient occupancy to track dispersion of the virus inside and directly outside the room, to better inform infection prevention and control procedures in the hospital. We decided to sample patients, their healthcare providers and their rooms over time, tracking both the virus and the microbial communities.
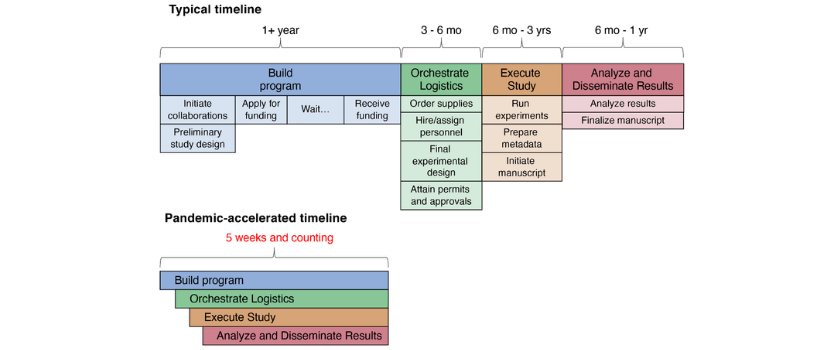
Usually when planning a scientific study, we have ample time – months, if not years – to line up the necessary pieces. We start planning the details during the grant proposal process, laying out the rationale for each experimental choice and beginning to sketch out the details of how data would be generated and analyzed. Many months later, maybe years later if the grant is submitted multiple times, the funding award is announced and we have time to hire people, attain supplies and plan the experiments in more detail before the start date. In the face of a global pandemic, where answers are urgently needed and the unknowns are overwhelming, we had to get the pieces in place quickly while keeping our lab members safe and communicating with an ever-growing network of collaborators under constantly changing circumstances. Like our lab, many other labs across the country and around the world have shifted their focus to COVID-19 research on a short timeline. Every university and lab likely has different processes and regulations, but here we will discuss some of the things we needed to get in place quickly to carry out the study, such as approvals, safety precautions and certifications, adapting our essential personnel to changing needs and ensuring that carrying out the experiment would not negatively influence clinical care or occupational health and safety.
Supplies
Since studies began immediately prior to the sudden acceleration of the pandemic in the USA, we were able to secure sample collection materials and reagents that would allow us to detect the virus. Our research-grade SARS-CoV-2 RNA detection reagents are comparable to clinical grade materials, without competing with healthcare provider needs. Many companies are prioritizing orders specifically for COVID-19 research, so we have been in close contact with our sales representatives. In part due to the fact that all non-essential non-COVID-19-related research has been halted, we have been able to reallocate supplies from other projects. We are also testing alternative methods and smaller reagent volumes to stretch supplies as far as possible to enable project longevity.
Institutional Review Board (IRB)
IRB approval is a requirement for any studies involving data from human subjects. We were able to leverage existing IRB protocols, consent forms and questionnaires from ongoing studies to begin the study immediately. The IRB was incredibly fast and responsive to changes we have had to make along the way, such as including new sample types, getting consent from family members for patients who were unable to consent for themselves and linking patient samples to environmental samples. Any change in the study has to be in line with what the IRB approves, so throughout the past few weeks as experiments have evolved, we have gone through the expedited amendment process several times.
Biohazard Use Authorizations (BUA)
BUAs dictate what sample types are approved to be received and worked with in a given laboratory. Our laboratory personnel are highly experienced in receiving human biological and environmental samples but had not previously received samples likely contaminated with an identified highly infective virus. We wrote our sampling protocols so that the virus would be inactivated immediately after collection by submersion in 95% ethanol. University policy of how these samples are classified has changed over the past 5 weeks so, like our ongoing efforts with the IRB, we have been in constant contact with our BUA coordinator. We have also enhanced our cleaning and sanitation protocols and added additional personal protective equipment requirements (albeit without impacting clinical needs), while frequently checking for updates to the recommendations.
 The Microsetta Initiative in the time of COVID-19
The Microsetta Initiative in the time of COVID-19
Daniel McDonald and Jack Gilbert discuss the Microsetta Initiative and how it evolved to help in the fight against SARS-CoV-2.
Personnel
We already had an extended network of labs that frequently collaborate, but with the urgency of COVID-19, multiple labs have essentially fused together. This huge pool of talent makes everything easier but adds some challenges. For example, we had to make sure that people from different labs were approved to work in each other’s physical lab spaces, and that everyone involved in the project was up to date on required certifications for work in each lab and with these sample types. We set up a rotating ~6-person crew to securely transport samples from the hospital, who all required training on the transport of biosafety level 2+ samples and dry ice. For sample processing, we pared down the number of people needed to be physically on campus to the bare minimum, so as to practice social distancing even in the lab, and had these people classified as essential personnel. We check in frequently with everyone working in the lab and transporting samples, asking if they are still comfortable working in person and assuring them that they can, at any point, choose to stop.
Not getting in the way
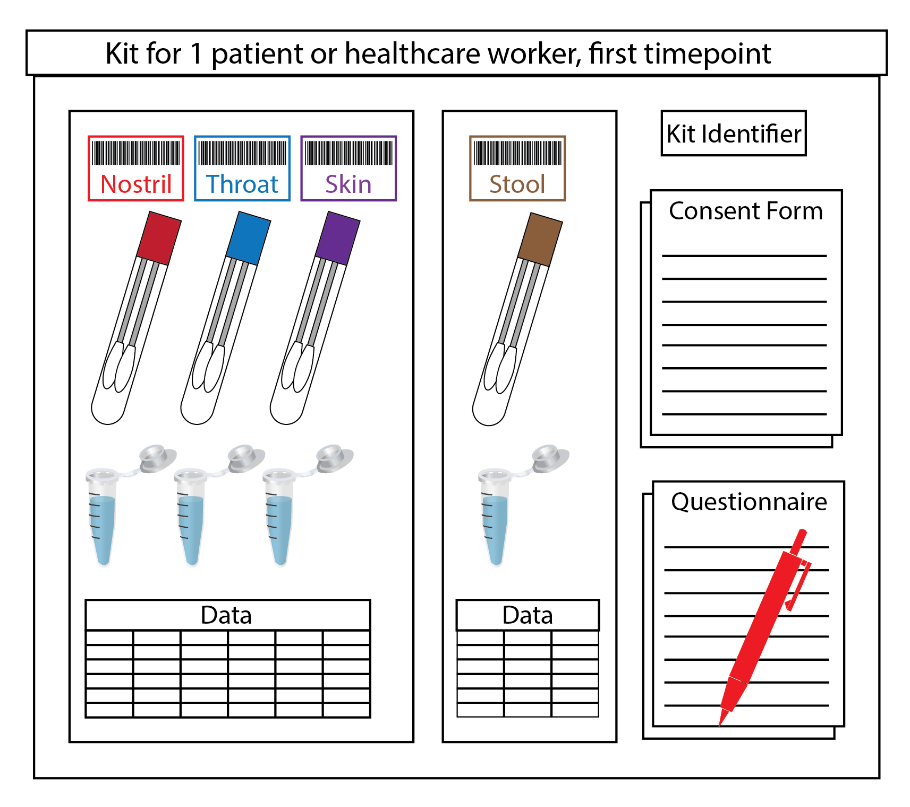
Although we believe that this project can have enormous benefits and could help shift national policy for infection prevention and control and potentially save lives, we also want to ensure that sampling does not interfere with patient care. To address this, we streamlined the sample collection process as much as possible so that it can be completed quickly by a doctor already in the room providing patient care. This puts the smallest possible burden on the doctor’s time and personal protective equipment, which is critically in short supply in many hospitals. On the lab side, it also makes things a little more time-consuming, as we spend a lot of time preparing kits to be prelabeled and as safe as possible to transport and receive. Everything inside the containers coming from the hospital is considered infectious and is processed inside a BSL2+ biosafety cabinet, even paper questionnaires and data tables. Additionally, everyone in the lab adheres not only to standard environment, health and safety protocols for this facility, but also to enhanced social distancing practices.
In these unprecedented times, the unexpected is to be expected. In getting this project up and running, we benefited from existing systems that could be modified instead of requiring us to construct everything from scratch. For example, we are able to use an existing barcoding system to label sample tubes and track metadata. Our amazing clinical research fellows – doctors who are taking time post-residency to conduct research in our labs – have access to the hospitals and the appropriate training to help with sample collection and transport. Even with these helpful systems in place, we have had to adapt constantly over the past several weeks. As new urgent project ideas are developed, we carefully amend IRBs and BUAs, order supplies, design sampling kits and write protocols and grant proposals. As people around the world stream TV and movies and conduct video calls at an unprecedented rate, we prepare for potential bandwidth limitations that might affect our data-intensive workflows. As some methods work out and others don’t, we check in with our growing network of collaborators, sharing information, supplies, samples and anything else that might help. And as we take part in this collaborative global effort to mitigate the trajectory and deadly effects of the pandemic, we are reminded once again that we are all in this together.