Three non-blind mice
Researchers prevented blindness in mice using CRISPR/Cas9-mediated therapy.
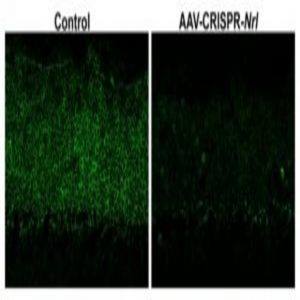
(Left) Retinal photoreceptor layer after control treatment with AAV-CRISPR/Cas9 against EGFP. (Right) Retinal photoreceptor layer after 2.5 months of treatment with AAV-CRISPR/Cas9 against Nrl showing drastically reduced Nrl expression.
Credit: Wenhan Yu, National Eye Institute, Bethesda, MD
Your ability to read this article comes courtesy of two hardworking light-sensitive receptors in your eyes: rods, responsible for night and peripheral vision, and cones, responsible for daytime and color vision. In humans suffering from the genetic disorder retinitis pigmentosa, the rods degenerate, which then triggers degeneration of the cones. Symptomatically, patients suffer from loss of night and peripheral vision at the onset of the disease, leading to complete blindness over time.
Based on previous studies (2), Wenhan Yu from the National Eye Institute in Bethesda, MD knew that preventing rod degeneration could rescue the cones and prevent blindness—but how to protect the rods in live animals remained a mystery. Now, Yu and his team report in the journal Nature Communications that by using the precise gene editing tool CRISPR/Cas9, they have prevented blindness in mice suffering from retinitis pigmentosa (1).
Targeting the individual disease-causing genes was challenging, since more than 60 genes are involved in the different forms of retinitis pigmentosa. However, “Most of the disease causative genes are rod specific genes,” Yu said. So the team chose a broadly applicable approach and targeted the Nrl gene, which encodes a transcription factor that controls expression of several genes crucial for rod function.
First, they packaged CRISPR/Cas9 machinery designed to introduce deletions or insertions in the Nrl gene into an adenovirus vector (AAV) delivery system and injected it into the retinas of healthy adult mice. Genetic tests verified that the Nrl mutation drastically reduced expression of rod-related genes in the photoreceptors.
The team next injected their AAV-CRISPR/Cas9 system into three types of mice suffering from retinitis pigmentosa caused by different genetic mutations. Over the course of 3 months, they saw a larger number of viable rods in the CRISPR/Cas9-treated mice than in those without treatment. The presence of alive—albeit dysfunctional—rods promoted cone survival in the treated mice, thereby preventing blindness. “This is a first experiment using AAV-CRISPR/Cas9 to knockdown Nrl as a universal strategy to treat retinitis pigmentosa,” said Yu.
Stephen Tsang, an ophthalmology researcher at Columbia University, found these results promising for exploring future therapeutic options but expressed concern regarding the approach. “They are trading one disease with another disease, hoping that the second disease is better,” he said, referring to night-blindness and other possible deleterious effects induced by intentionally shutting down rod function in the Nrl-mutated mice.
Yu hopes that the AAV-CRISPR system will serve as a platform for knocking down different genes for further studies on disease progression. While there is some potential for eventually using this approach in humans, he cautions that those are long term goals since the safety of this approach has not been proven.