Neurobiology’s model revolution

From patient-derived induced pluripotent stem cells to organoids, neurobiologists have new tools for their experiments. Nathan Blow looks at how this is leading to the next step in brain research.

The discovery of induced pluripotent stem cells (iPSCs) traces back to 2006, when Shinya Yamanaka, then at Kyoto University, first reported a method for using four specific transcription factors to revert adult cells back into a pluripotent stem cell state. These cells became known as iPSCs, and for his discovery, Yamanaka won the 2012 Nobel Prize in Medicine along with Sir John Gurdon.
iPSCs have been instrumental in advancing the work of many stem cell researchers, since these cells bypass the ethical issues associated with using human embryonic stem cells. But there is another notable advantage to using iPSCs in research; since they are derived from specific adult cells, it’s possible to create patient-specific iPSC lines that can be differentiated into various cell types in the lab.
Following Yamanaka’s discovery, scientists from around the world labored to refine the techniques for deriving iPSCs. Today, there are improved protocols and methods for generating these cells from adult cells and also for differentiating them into various cell lineages. So, it comes as no surprise that their use for disease modeling has exploded in recent years.
Turning Back the Clock
At the moment, the genetic and molecular underpinnings of autism spectrum disorder (ASD) remain unclear. While researchers are starting to pinpoint some potential causes among patients, ASD has proven to be a complex condition. Still, scientists remain keen on uncovering a clear explanation for ASD development and progression.
In late 2017, Fabiele Baldino Russo from the University of Sao Paulo and colleagues reported another step toward understanding ASD when they created a cell model of the condition using ASD patient-derived iPSCs (1). To accomplish this feat, the researchers first derived iPSCs from three individuals with non-syndromic ASD as well as from three control subjects. From there, the authors differentiated the iPSCs into patient-specific astrocytes and neurons to examine and compare various cellular functions, including protein levels, glutamate neurotransmitter release, and spontaneous firing rates.
Russo and colleagues’ data showed clear differences between cells derived from ASD patients and control cells. In particular, ASD-derived astrocytes interfered with the proper development of neurons when the two were cultured together; this could be rescued by using control-derived astrocytes instead. Taking advantage of the patient-derived brain cells, along with advanced culturing techniques, Russo’s team teased out new insights into how ASD develops.
Cell studies like these would not be possible without the use of stem cells. But ASD is not the only condition researchers are using iPSCs to model—and culturing different cell types together is not the only way researchers are applying iPSCs in their experiments.
Model Behavior
By most accounts, schizophrenia symptoms usually first manifest in patients during their adolescent years. Symptoms can include delusions, thought disorders, hallucinations, paranoia, and depression, to name a few. But according to scientists, the disease most likely originates much earlier in life.
Current research suggests that brain structure changes in individuals with schizophrenia occur as early as the end of the first trimester in uetro. To better understand how such structural changes impact schizophrenia progression, researchers from the Icahn School of Medicine at Mount Sinai and the State University of New York (SUNY) at Buffalo used iPSCs to examine molecular and genetic events taking place during early development in schizophrenia patients (2).
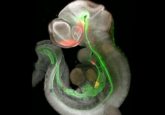
The research team, led by SUNY-Buffalo professor Michal Stachowiak, first generated iPSCs from patients with schizophrenia as well as from a control group. They then used these cells to create small, three-dimensional balls of cells the size of a bean, called cerebral organoids, that recapitulate key aspects of brain development during the first and second trimesters of pregnancy.
“We mimic this process in the laboratory with stem cells, focused specifically on developing the cerebral organoids that resemble the developing human brain in its earliest stages of growth,” explained Stachowiak.
The method for generating cerebral organoids was actually developed several years ago by a team of scientists led by Madeline Lancaster and Juergen Knoblich at the Institute of Molecular Biotechnology of the Austrian Academy of Science. Lancaster, Knoblich, and their colleagues’ method for culture of 3-D organoids, which they called cerebral organoids, can be used to develop discrete and independent brain regions in a lab dish (3). These organoids demonstrated many of the features associated with regular human cortical development and have since been used in several studies, including Stachowiak’s schizophrenia work.
Stachowiak’s results showed that schizophrenic patients had an abnormal distribution of proliferating stem cells outside expected brain regions, curtailed neuronal development in other regions, and a lack of expression of nFGFR1—a gene crucial for governing gastrulation and subsequent body and nervous system development. To verify the role of nFGFR1, the authors actually blocked and depleted FGFR1 in a control cerebral organoid and found similar cortical developmental arrest patterns to those seen in schizophrenia-derived organoids.
“We now can state that schizophrenia is a disorder of faulty brain construction that occurs early in development, corresponding to the first trimester, and involving specific malformation of neuronal circuits in the cortex,” Stachowiak concluded.
Brain-machine Interfaces and Beyond
Stachowiak’s group is now taking their cerebral organoid setup in a slightly different direction. “We are working on combining the organoid research with smart nanophotonic devices to develop a new generation of brain–machine interfaces,” explained Stachowiak. “With this technology, one may eventually be able to control and correct development of cells in the complex tissue of the developing brain. An important step toward developing such technologies will be testing them in cerebral organoids, or mini-brains, to see if they can actually direct and modify the developing brain in real time.”
As it turns out, Stachowiak is not alone in his quest to create interactions between brain organoids and machines. The emerging field of neuroprosthetics seeks to use electrical devices to stimulate and record neural circuits from within the brain. While still in the earliest stages of development, the opportunity to use cerebral organoids in the testing of new neuroprosthetic devices should allow researchers to examine neurons, neural connections, and brain structures in unique ways.
Beyond being able to interface machines with brain organoids, other groups have recently demonstrated the possibility of fusing organoids derived from different stem cell populations, creating neural networks possessing more sophisticated features and cellular interactions characteristic of the human brain. Up to this point, the majority of brain organoid research has been in vitro, on the lab bench in dishes. But organoids made headlines in November 2017 when two research groups presented data on the in vivo implantation of brain organoids. At the recent Society for Neuroscience meeting in Washington, DC, both research teams described implanting human stem cell–derived brain organoids into the brains of mice. They reported that the organoids integrated with mouse brain tissue and, in one case, actually grew for two months.
Advances in iPSC and organoid studies, along with the emergence of neural engineering efforts, are giving life scientists and the general public further hope that stem cells will prove to be informative models for the study of brain development and function, not just in the short-term, but for many years to come.