Oxygen deprivation boosts immunity
Short spells of hypoxia help the immune system combat later infections.
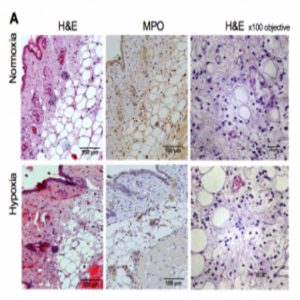
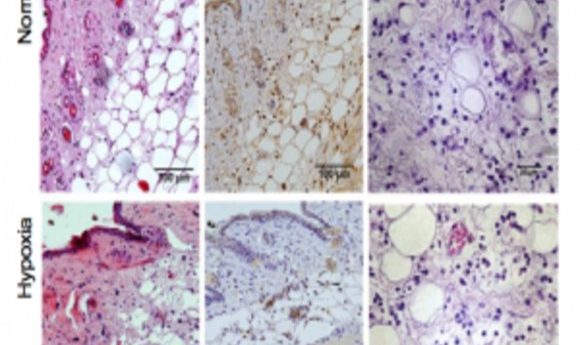
Local immune responses are not impaired by hypoxia (1).
People experience a lack of oxygen in body tissues in lots of health conditions, from melanoma to lung disorders. Thanks to earlier research, researchers know that deficient oxygen levels induce inflammation and also occur in inflamed tissues due to higher body-oxygen consumption during infection. But is hypoxia always harmful?
Writing in Science Immunology, scientists report that hypoxia actually preconditions the innate immune response to improve survival after bacterial infection.
“This adds a temporal dimension to hypoxic response, [which] is important when considering progressive responses to tissue damage and infection,” explained co-author Randall Johnson at the University of Cambridge.
To find new ways of combating the deadly results of hypoxia in infection, senior author Sarah Walmsley at the University of Edinburgh and her colleagues studied the results of hypoxic “doses” in mouse models of chronic hypoxia that developed Staphylococcus aureus and Streptococcus pneumoniaeinfections.
Short bouts of hypoxia during infection allowed mice to fight the bacteria but also decrease survival rates, due to the effects of fever, weight loss, or heart damage. In contrast, longer pre-infection hypoxic exposures disrupted the hypoxia-inducible factor (HIF) pathway and modified glycolysis genes, protecting cardiac cells and the body’s energy balance after infection set in.
“Our work suggests that actually what’s causing the damage is not failure to control the bacteria, but the disorganized or exuberant inflammatory response that occurs in the setting of hypoxia,” said Walmsley. “We need to be developing strategies that target the immune response in parallel with required antimicrobials.”
During infection, neutrophils require glucose in order to mount a pro-inflammatory response, “but that actually becomes a detriment in acute hypoxia because it starves the rest of the animal,” said Walmsley. Pre-infection hypoxia treatment gave the neutrophils a “memory” of hypoxia, preparing them for future oxygen deprivation.
The team linked this improved survival to bone-marrow white blood cells, which required less glucose and, thus, protected the mice from nutrient depletion. “That resulted in the suppression of the HIF pathway and reprogrammed leukocyte metabolism,” explained Walmsley.
“It’s great to see basic theories come alive in functional models such as the one used by Sarah’s group,” said Darren Richard at Université Laval, Québec. “Even if we’ve been working on hypoxia for several years, there are still a number of important breakthroughs to come!”