Common gut microbe counters metabolic syndrome
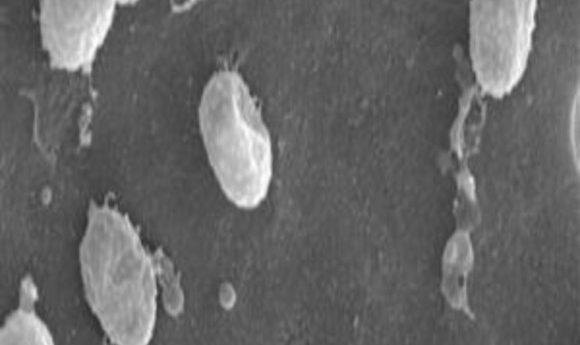
Pasteurized bacteria or a single microbial protein can reduce the symptoms of metabolic syndrome and obesity in mice. Human studies to test the probiotic are under way.
Akkermansia muciniphila
A protein made by a common human gut microbe improves the symptoms of metabolic syndrome and obesity in mice, according to a new study published in Nature Medicine. In an ongoing clinical study, researchers are testing whether the microbe’s benefits are also seen in overweight people.
The bacterium in question, known as Akkermansia muciniphila, has long been linked to better cardio-metabolic health in healthy individuals’ gut microflora, and its proportions are lower in diabetic or obese people. In several previous studies, researchers found that treating rodents fed a high-fat diet with A. muciniphila reduced weight gain. But extending those experiments to human subjects was tricky.
- muciniphilacan only be cultured in a growth medium containing mucus, and human use as a probiotic requires a food-grade sample. Because high temperatures destroy the microbe’s proteins, Patrice Caniof the Catholic University of Louvain in Belgium and his colleagues turned to pasteurization—the process used to render milk, cheese, and other foods safe to eat. The team heated bacterial cultures to 70o C for 30 minutes to kill the microbes, then tested whether the pasteurized bacteria retained their beneficial effects. “Our aim was to translate the rodent data into human applications,” Cani said.
They found that when mice were placed on a high-fat diet, animals that received live bacteria experienced 40%-50% less weight gain than those that were fed the fatty diet without the probiotic. However, pasteurized bacteria exerted an even stronger effect. Mice fed a high-fat diet with pasteurized A. muciniphila cultures only gained as much weight as animals on a normal diet.
Rodents treated with pasteurized cultures showed lower insulin resistance and better glucose tolerance. Their fat cells were smaller in size, and levels of plasma leptin—a hormone secreted by fat—were significantly lower, an effect not seen with the live bacteria.
“We were really surprised to find that the bacteria were still active when pasteurized and even more potent than when alive,” Cani said. “We still don’t know all the reasons why.”
Pasteurization may remove some inhibitory enzymes, or the process may render certain especially potent proteins more accessible, he suggested. Using genomic and proteomic analysis, the researchers found that Amuc_1100, a bacterial membrane protein, was among the most abundant molecules and could interact with Toll-like receptors on human cells. When they tested its effects on cell cultures and in animals, the isolated Amuc1100 protein worked much like the pasteurized bacterium.
“We knew based on the proteomic analysis that Amuc_1100 was highly abundant, but I was not really convinced that this one protein could explain anything until we saw the data,” Cani said. “We don’t yet know whether the protein Amuc_1100 is able to drive the beneficial effects alone or it’s doing it in synergy with other gut components.”
Although the purified protein will need to be tested in additional experiments, the researchers have already begun clinical trials to assess pasteurized microbes for safety and effectiveness. If the treatment proves beneficial, it could offer a new route to treating metabolic syndrome and obesity.