Metals help bacteriophages kill multi-drug resistant bacteria
Magnesium, calcium, and iron boost the antibacterial activity of phages in blood.
Strains of multi-drug resistant bacteria are emerging at an alarming rate, leading researchers to search for new ways to combat antibiotic-resistant infections. Bacteriophages, bacteria-eating viruses, are attractive options since they naturally target bacteria. A significant roadblock in developing phages as treatments for multi-drug resistant infections is that researchers don’t fully understand how phages behave in complex environments such as the human body.
“Phage therapy is a promising avenue for dealing with multi-drug resistant bacterial infections, and there is certainly evidence that it can work in animal model studies. And there is also promise from individual case studies in humans,” explained Jason Gill from Texas A&M University. “The thing is, when it doesn’t work, usually little work is done to figure out why,”
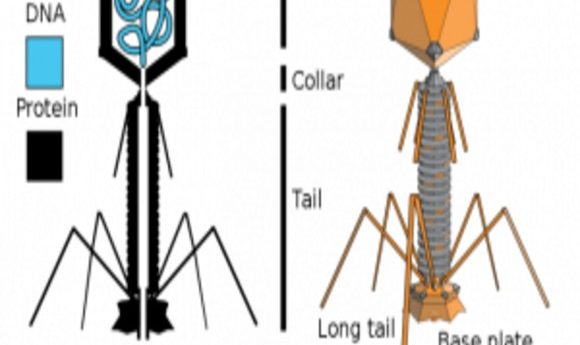
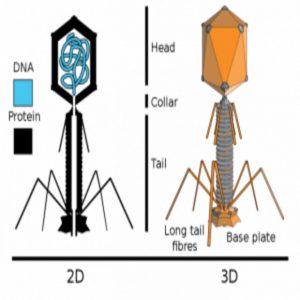
In a new study published in Scientific Reports, Anthony Maresso and his colleagues from Baylor College of Medicine looked at the ability of phages to kill a multidrug-resistant form of Escherichia coli in human blood. They found that certain metals enhance bacterial killing by bacteriophages in blood.
The team began their study by treating ExPEC, multidrug-resistant extra-intestinal pathogenic E. coli, with phage in blood that was treated to remove innate anti-bacterial components as well as in bacterial culture medium. They found that the phage killed bacteria in the culture medium but not in the blood.
The blood samples contained ethylene diamine tetraacetic acid (EDTA), a metal ion chelating agent that prevents clotting, so the authors repeated their experiments using heparin, a natural anti-coagulant. To their surprise, bacterial killing was slightly more efficient in heparin-treated blood than in EDTA-treated blood.
“The EDTA thing pointed us to metals,” said Maresso.
The team next tried adding three divalent cations, calcium, magnesium, and iron, which are commonly found in human blood and tested phage activity against ExPEC. In heparin treated blood, calcium, magnesium, and iron added individually or in pairs enhanced phage-dependent killing of bacteria approximately 10,000-fold. In EDTA-treated blood, calcium and magnesium increased killing of bacteria.
Further experiments showed that adding metals enhanced killing of other strains of E.coli as well. Metals also enhance ExPEC killing in mice with localized ExPEC infections.
“Our results are some of the first to demonstrate that the ability of phages to bind to bacteria, replicate, and kill bacteria are different in standard microbiological media and in the blood mimic we use. This important observation means we cannot simply assume that phage infections are identical in the lab and in an individual receiving phage therapy,” said Robert Franklin Ramig, a professor at Baylor College of Medicine and co-author of the study.
While this is a positive first step, Maresso cautioned that there are many things left to consider before developing therapeutic bacteriophages. For example, the number of phages that one can use is unlimited, so scientists need to screen phages to determine which work best in therapeutic settings. “We also need to know what other factors in the blood can negatively or positively affect phage activity,” explained Maresso.
“We expect that that these studies will affect development of phage therapies because they demonstrate phage properties are different in vitro in microbiological lab media and in vivo treated patients,” added Ramig.
Gill agrees, clarifying that using phages in vivo brings a lot of other factors into play. “I don’t know how easy it is to modulate things like cation concentrations in vivo without causing other complications, but it might mean that another criteria for selecting therapeutic phages will be their cation dependence,” said Gill who was not involved with this study.