An ultrasound peek into live cells
Want to observe the inner nanomechanics of cells without interfering with normal biology? This new ultrasound bioprobe may be the solution.
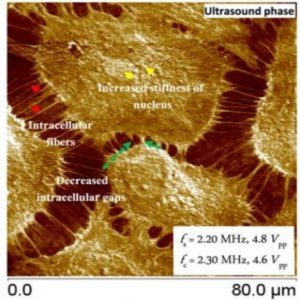
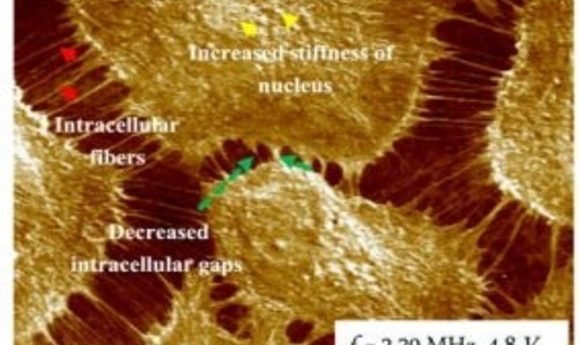
Ultrasound bioprobe phase image (1).
Imaging is a cornerstone of cell biology. Despite the numerous cell imaging technologies available, scientists still crave a single highly sensitive method with excellent spatial and temporal resolution. “The problem with the confocal or these fluorescent microscopes is the resolution doesn’t go down to less than 100nm,” said Gajendra Shekhawat from Northwestern University, first author of a new study published in the journal Science Advances. Many fluorescent contrast imaging methods also require staining processes that can also unnecessarily modify cell properties.
Hoping to fill the void, Shekhawat’s team developed an ultrasound bioprobe that can image live cells at the nanoscale level with high resolution, overcoming some constraints of current imaging methods. “What this technique will allow scientists to do is image those nanostructures within those cells down to 5-10 nm without any stain,” he explained.
This ultrasound bioprobe combines the strength of ultrasound imaging with atomic force microscopy (AFM) to image live cells without damaging them. Traditional AFM uses a mechanical probe to detect the sample. For using the new bioprobe, Shekhawat’s team designed a setup that emits ultrasound waves from below and above the sample. When the waves hit the sample, the subsurface contact is recorded by the AFM probe. To reduce acoustic dampening that often occurs in fluids and improve their imaging, the researchers also built in a feedback mechanism.
“They are localizing their ultrasound imaging to a very fine point and then using this native particle contrast agent to really dramatically increase the spatial resolution. So to me, that is the neatest thing about this paper,” reflected Jesse Jokerst from the University of California, San Diego, who was not involved in this study.
To test the technology, Shekhawat’s team designed a “cell” made up of a silica membrane covered in receptors approximately 5-10 nm in size, and with a magnetic core embedded inside. Spread across a mica substrate, the diameters of these silica “cells” averaged around 30-40 nm each. Standard AFM imaging only detected the silica outer layer, while another more traditional microscopy method only imaged the embedded core. Neither could detect and distinguish the different components of the synthetic “cell” well. However, all three structures appeared in the phase contrast image and from the ultrasound bioprobe. The contrast varied based on the material’s stiffness, with brighter contrast coming from stiffer content.
“In this case, we can measure, basically, the biomechanics of cellular structures—of subcellular structures. That can have a wide range of applications,” Shekhawat said.
Shekhawat next used the ultrasound bioprobe to image live endothelial cells. Understanding the subcellular nanomechanical mechanisms of fiber formation in these cells may aid in understanding and treating acute lung injury and acute respiratory distress syndrome.
The researchers treated the cells with thrombin to stimulate fiber growth. The ultrasound bioprobe imaging before and after thrombin treatment showed extreme contrast of intracellular fibers that stretched gaps while subcellular phase contrast focused on the nuclear area. Using this technology, Shekhawat could detect very small intracellular fibers along with the subsurface stiffness contrast of thicker and smaller fibers.
“It has a lot of potential. I think what is nice is that AFM is an already established technique. Many universities’ research labs already have an AFM, so it could be relatively easy to adopt,” Jokerst commented.
Shekhawat now plans to use the ultrasound bioprobe to understand how drugs impact endothelial cells, what types of molecular fiber repair occur, and how drugs are transported in nanoparticles. “It will open up whole [new] applications for other scientists to explore with this technique,” he concluded.