Mighty muscle myosin
Myosin in muscles behaves in a unique way, which might explain why muscles have such strong contractile power.
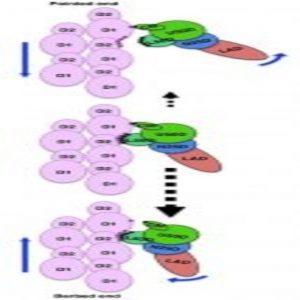
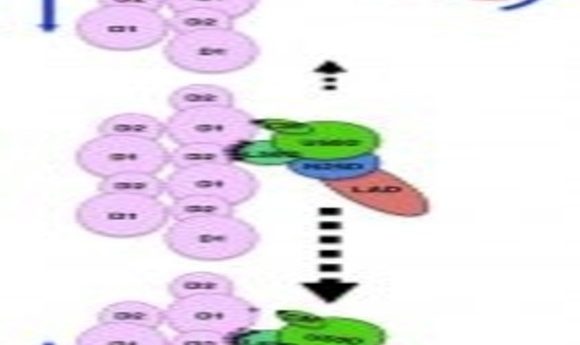
A cartoon showing the asymmetry in the weak binding between myosin and actin that biases the direction of their dissociation.
Credit: Osaka University
Researchers love to use cartoons to demonstrate what’s taking place inside a cell. Some of the most delightful of these cartoons illustrate the interactions between actin and myosin. With a blob for a head and a long tail, myosin reaches out to grab onto actin, gives it a sharp tug, and then bounces forward to grasp actin further along. In this way, it travels along actin when transporting cargo within a cell or contracting a muscle fiber.
Even though researchers have extensively studied actin and myosin over the past 50 years, some mysteries remain regarding their interaction. For example, the myosin head is an ATPase that rapidly separates Pi and ADP, but these products stay attached to myosin. Structural studies never quite resolved how ADP and Pi are released or how ATP binding severs the actin–myosin connection. And then there is the fact that myosin seems to travel further with each pull than expected based on its structure. “There were no atomic images of ATP hydrolysis when myosin is interacting with actin,” said Takashi Fujii from Osaka University in a press release.
Now Fujii and Keiichi Namba report a 5.2-A resolution cryoelectron microscopy structure that shows myosin interacting with actin in the journal Nature Communications. By comparing their structure with structures of myosin in various states, the authors uncovered a previously unknown conformational change in myosin during its interaction with actin, which they believe explains why muscle myosin has faster kinetics than other myosin proteins.
The electron microscopy data confirmed that each myosin head interacts with two actin molecules at three major binding sites, coordinated mainly by hydrophobic and electrostatic interactions. In response to ATP binding, myosin rotates, reducing the number of connections with actin. This weakened connection allows myosin to release actin. It then hydrolyzes the ATP and rebinds further along the actin molecule, thus pulling the filament. The team showed that ADP and Pi could theoretically exit in either direction during mysosin’s conformational change.
“This is a very unique image because the weak binding state is unstable, and its lifetime is short,” explained Namba. “There is a structural asymmetry in the system. This could explain why myosin moves over much longer distance per ATP hydrolysis than expected.”
In addition to providing a new update for the actin–myosin cartoon, the team plans to use its observations for building machines. “We are studying nature’s nanomachines to build man-made ones,” Namba said.