Parkinson’s disease and the human olfactory bulb

Problems with olfaction are one of the earliest symptoms of Parkinson’s disease—yet more attention is paid to its motor issues. Now, researchers are looking closer at the human olfactory bulb to better understand what may be going awry.

Mention Parkinson’s disease, a neurodegenerative disorder that affects approximately half a million Americans, and people think of the disorder’s hallmark motor symptoms: muscle weakness, difficulty walking, and tremors. But most patients also exhibit a distorted or absent sense of smell.
“Parkinson’s disease was described precisely 200 years ago by Dr. James Parkinson, but it took until 1975 for olfactory dysfunction to be recognized as an important part of the disease,” said Peter Mombaerts, a researcher at the Max Planck Institute in Germany. “Ninety percent of Parkinson’s patients suffer from olfactory dysfunction, but not every person with a diminished sense of smell will develop Parkinson’s. In our view, it was not clear what was wrong with the olfactory system in humans and how it might relate to the disease.”
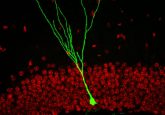
In 1996, Mombaerts and Richard Axel at Columbia University created a novel technique for visualizing the axons from olfactory sensory neurons in mice as they project to the glomerulus, a specialized structure in the olfactory bulb where synapses with second-order neurons form, allowing smell information to be integrated and processed. This approach had not been used to study human olfactory bulbs.
“If one wanted to search for possible anatomical explanations for olfactory dysfunction in disease, focusing on the glomerular component of the olfactory bulb is a good place to start,” Mombaerts said.
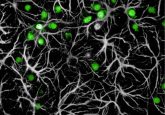
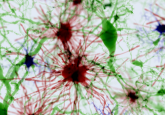
To do so, Mombaerts and his colleagues took post-mortem sectional samples of the olfactory bulb from six healthy individuals and five Parkinson’s disease patients. Then using fluorescent immunohistochemistry, they made a three-dimensional reconstruction of the entire olfactory bulb, which revealed that the global glomerular volume in Parkinson’s patients was half that of healthy individuals and had a very different distribution of the glomeruli across the bulb.
“When the volume taken up by the functional units of the olfactory bulb is halved, it’s likely that this strong reduction, in and of itself, could explain olfactory dysfunction,” Mombaerts said. “Unfortunately, we don’t have information about olfactory performance in these particular cases so we cannot say for sure.”
Future longitudinal, prospective studies that assess olfactory function during life and then compare post-mortem reconstructions as done in this study should help scientists to better understand Parkinson’s pathology as it relates to the olfactory bulb and may offer critical new insights into the progression of the disease.
“We need to explore further abnormalities in the olfactory system. What is the vulnerable cell type in the olfactory bulb of Parkinson’s patients?” Mombaerts asked. “There must be certain cell types affected, similar to the well-described pathology of dopamine neurons in the substantia nigra. So this reinforces the need for more research on the pathology in the olfactory system of Parkinson’s patients so we can better understand what is happening with the disease.”