Diabetes and the gut microbiome
A new study reveals a critical role for intestinal flora in preventing disease.
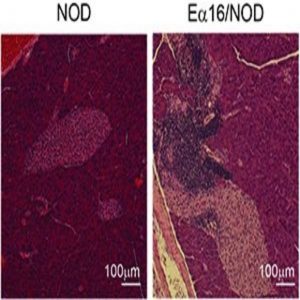
Pancreas sections showing islet inflammation, or insulitis, in NOD mice that spontaneously develop type-1 diabetes (left) or lack of insulitis in Ea16/NOD mice that do not develop diabetes because they carry a protective H-2 allele (right).
Credit: Diane Mathis
In type I diabetes (T1D), autoimmunity destroys insulin-producing pancreatic islet beta cells, usually beginning in childhood or adolescence. Although genetics play a role in T1D, there are also important environmental influences. (Identical twins have less than a 50% co-occurrence of T1D, and incidence of the disease has increased in recent decades.) Now, scientists report a link between a specific genetic component of T1D and the maternal gut microbiome.
“Protective…alleles have been identified for T1D in both humans and mice. They (but not necessarily the same ones) have been identified in a number of other autoimmune diseases, which have also been shown to be under the influence of the microbiota,” said Diane Mathis of Harvard Medical School, one of the study leaders.
Mathis and her colleagues study non-obese diabetic (NOD) mice, which spontaneously develop autoimmune diabetes characterized by leukocyte infiltration of pancreatic islets (insulitis). For this study, they turned to Ea16/NOD mice, where the defective allele in NOD mice is complemented with a normally expressing allele, protecting them from insulitis and diabetes.
Mathis’ team observed that NOD progeny born to Ea16/ NOD mothers mated with NOD fathers, had far fewer insulitic islets and less frequent diabetes when compared with NOD progeny born to NOD mothers mated with Ea16/NOD fathers, suggesting a maternal effect. In addition, earlier work by Mathis’ group showed that the microbiome can prevent NOD mice from developing diabetes.
To investigate a possible role for the mother’s microbiome in T1D, the researchers treated pregnant Ea16/NOD mothers with vancomycin, a broad-spectrum antibiotic, and found increased insulitis among the progeny. When they performed 16S rRNA sequencing of the fecal microbiome in 3-week-old pups from treated Ea16/NOD mothers, they confirmed a shift in the bacterial phyla present when compared with untreated controls.
Ea16/NOD progeny are not insulitic, but the team found that treating pups from 6 to 6 weeks of age with vancomycin resulted in insulitis, with the greatest effect showing up around 3 weeks of age, the approximate age of T1D onset in mice.
Mathis believes that structural differences in the MHC II molecules of NOD mice make them susceptible to T1D through interaction with the gut flora. “There are differences, especially in the parts of the MHC molecule important for binding antigenic peptides. So the different alleles could permit different types of immune responses to different bacterial antigens and promote or inhibit their establishment in the gut,” she said. Mathis and her colleagues are now trying to narrow down which bacteria are responsible for T1D protection.