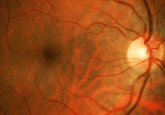
Understanding Alzheimer’s disease: answering some frequently asked questions
Alzheimer’s disease is an age-related neurodegenerative disorder, characterized by progressive neuronal degeneration that leads to impaired cognition and memory.
Named after the first doctor to identify the disease, Alois Alzheimer, the term Alzheimer’s disease was first used in a textbook in 1910. However, records of people demonstrating the clinical symptoms of dementia have been around since Roman times. For more on the history of Alzheimer’s, view our interactive timeline feature.
The 2015 World Alzheimer Report – subtitled ‘The Global Impact of Dementia’ – found that worldwide, there is a new case of dementia every 3 seconds. As of 2015, 46.8 million people were living with dementia, a figure expected to almost double every 20 years. It was predicting that by 2030, dementia will have a worldwide cost of over $2 trillion USD.
To mark World Alzheimer’s Month (September), we will be answering some of the frequently asked questions about Alzheimer’s disease, providing further information about the clinical and pathological characteristics of the disease as well as delving into the current status of the search for a cure.
- Alzheimer’s disease vs dementia: what is the difference?
- What are the clinical features of an Alzheimer’s diagnosis?
- What is the pathology of Alzheimer’s disease?
- What is the amyloid hypothesis?
- What are the stages of Alzheimer’s disease?
- What are the causes of Alzheimer’s disease?
- How is Alzheimer’s disease treated?
- What does the drug development pipeline look like for Alzheimer’s disease?
- Can Alzheimer’s disease be prevented?
Alzheimer’s disease vs dementia: what is the difference?
Despite being used almost interchangeably by some, Alzheimer’s disease and dementia are not the same. Dementia is a syndrome, a collection of symptoms that clinically presents as impaired memory and cognition. Alzheimer’s is a disease with specific neuropathological and clinical features that can cause dementia.
Alzheimer’s is the most common form of dementia, accounting for approximately two-thirds of all dementia cases. This is followed by vascular dementia (20%) and Lewy Body dementia (15%).
What are the clinical features of an Alzheimer’s diagnosis?
Alzheimer’s disease can be characterized clinically by the progressive loss of cognitive function, changes in behavior and mood, and reduced capability to carry out the activities of daily living. There is no specific diagnostic test for the disease, though with advances in imaging techniques and blood-based biomarker detection, such tests may be clinically viable in the near future. Artificial intelligence also presents new opportunities and has been used to develop a cognitive test that can determine whether an individual has, or is at risk of developing, Alzheimer’s disease in just 5 minutes. However, these tests are currently in trial stages and it may be sometime before they are in regular clinical use.
Without the ability to test for a specific marker, clinicians will predominantly look at symptoms and patient history. Where tests are given, these will be to rule out other causes, such as stroke or tumor.
The primary symptom of Alzheimer’s disease is impaired memory and other symptoms of dementia. The 5 A’s of Alzheimer’s detail the five types of memory impairment most commonly experienced by dementia patients:
- Amnesia – loss of memories and, in some cases, difficulty forming new memories
- Aphasia – loss of the ability to express one’s self through speech
- Apraxia – difficulty performing skilled movement, though muscles and general motor function remain largely unaffected
- Agnosia – inability to recognize objects
- Anomia – inability to remember the names of objects, this differs from agnosia as how to use an object is remembered but there is a failure to verbalize it.
In addition to memory loss, patients may experience non-cognitive changes such as changes in mood, behavior or personality.
What is the pathology of Alzheimer’s disease?
Alzheimer’s disease can be characterized pathologically by the presence of amyloid plaques, neurofibrillary tangles and cerebral atrophy. While some of these can be visible on brain scans, in many cases the pathological diagnosis of Alzheimer’s cannot be given until postmortem. It is worth noting that these pathologies also occur in a normally aging brain, but to a lesser extent. The distinction between normal aging and an Alzheimer’s brain is based on the quantification of the lesions [1].
Amyloid
Amyloid-beta (Aβ) peptides are a group of peptides formed from the cleavage of amyloid precursor protein (APP) by the enzyme β-secretase. Aβ comes in many forms, though it is most commonly found in its two major forms: A1-40 and A1-42. A1-42 is a more neurotoxic form, as it aggregates faster and leads to the formation of plaques.
An amyloid plaque, or senile plaque, is a large extracellular deposit formed from a core of aggregated Aβ peptides and the debris of degenerated cells.
Soluble A1-42 oligomers in the synaptic cleft have been demonstrated to inhibit long-term potentiation – a process of synaptic strengthening that is important for learning and memory.
Tau
Tau is a protein that, under standard conditions, stabilizes the microtubules that form the cytoskeleton of the neuronal axon. This allows for axonal communication and transport of other factors along the axon from one end of the neuron to the other. Usually tau undergoes a cyclical process of phosphorylation and dephosphorylation that is coordinated by the activation and inactivation of glycogen synthase kinase 3.
In an Alzheimer’s brain, there is an imbalance between the phosphorylated and non-phosphorylated states and the tau stays phosphorylated for longer periods of time. This state of hyperphosphorylation results in the formation of neurofibrillary tangles. The tangles sit in the axon of neurons, disrupting axonal transport and cutting off contact between the axon terminals and the cell body. This leads to neuronal apoptosis and, with its surrounding cell dead, the ‘ghost tangle’ is left in the extracellular space.
Cerebral atrophy
Typically, the brains of patients with Alzheimer’s disease would show modest cerebral atrophy. However, there is a correlation between both brain weight and cortical thickness, and the extent of cognitive decline. An MRI scan of a brain with late-stage Alzheimer’s disease would show shrinkage of both the medial lateral temporal and the frontal lobe, as well as enlarged ventricles and hippocampal atrophy.
Cell death occurs for a variety of reasons, including (but not limited to):
- Neurofibrillary tangles preventing axonal transport
- Inflammation
- Buildup of neurotoxic Aβ peptides
- Excitotoxicity caused by excess glutamate
- Neurotrophic factor withdrawal – neurotropic factors are small molecules released by cells that support the growth, survival and differentiation of surrounding cells. Once one cell dies, the surrounding cells that were previously relying on its neurotropic factors also die.
The types of neurons affected correlate to the clinical symptoms of the disease. Loss of cholinergic neurons (neurons that are excited by the neurotransmitter acetylcholine) has the strongest effect on cognition, whereas disruption of the serotonergic pathways leads to mood changes. The cerebellum remains largely unaffected, hence the lack of motor symptoms in Alzheimer’s patients.
In addition to neuronal loss, existing neurons lose the vital connections to their neighbors. The remaining cells have fewer dendrites, dendritic spines or axonal boutons than healthy neurons, resulting in a reduced capacity to form synapses.
 A timeline of key Alzheimer’s disease milestones
A timeline of key Alzheimer’s disease milestones
What are the key Alzheimer’s disease milestones? Find out how far Alzheimer’s disease research has progressed using this interactive timeline, and how much there is yet to discover.
What is the amyloid hypothesis?
Now a controversial area for many neuroscientists, the amyloid hypothesis was originally proposed as a starting point for sporadic Alzheimer’s disease development, effectively stating that amyloid causes Alzheimer’s and is the initial trigger for all of the other pathologies. This hypothesis was the basis for much of the academic and pharmaceutical research that has been carried out over the last 30 years; if amyloid causes Alzheimer’s, surely drugs that remove it from the brain will cure it?
Unfortunately, all therapeutics that have been developed under this principle have failed in clinical trial and none have ever been approved for general use. This, alongside additional research into alternative theories, has created doubt against a theory once held up as gold standard.
While some trials of Aβ antibodies have effectively slowed the progression of cognitive decline, the cure that many were hoping to find has remained elusive. It has become increasingly clear that Alzheimer’s is a multifaceted disease and therefore any potential treatment will need to take that into account, moving away from a purely amyloid-centric approach.
What are the stages of Alzheimer’s disease?
The decline in cognitive ability is characteristic of Alzheimer’s disease and the progressive exacerbation of clinical symptoms is mirrored by a gradual expansion of neuronal degeneration that spreads throughout the brain following a predictable pattern. This spread was first described in 1991 by German anatomist Heiko Braak. It has henceforth been known as Braak staging [2].
What are the causes of Alzheimer’s disease?
There are two main types of Alzheimer’s: sporadic and familial.
Familial Alzheimer’s accounts for between 5 and 10% of all Alzheimer’s cases and is where the disease is caused by an underlying genetic mutation. This form is heritable and predominantly results in early-onset Alzheimer’s, with symptoms starting before the age of 65. There are multiple different mutations that have been linked to familial Alzheimer’s disease and they are usually autosomal dominant, meaning only one allele is needed for the phenotype to have an effect. Genes that can be mutated include APP, PSEN1 and PSEN2 [3].
Sporadic Alzheimer’s disease is less likely to do with a single genetic mutation and is more due to a mixture of both genetic and environmental risk factors. The risk factors for developing Alzheimer’s disease are cumulative, therefore the more factors one has the greater their risk of developing the disease [3].
A key risk factor for developing the disease is the presence of one or more copies of the ApoE4 allele. ApoE is produced by astrocytes and is responsible for the removal of amyloid-beta from the brain. The allele ApoE4 is a risk factor for Alzheimer’s as it causes the initially protective role that ApoE plays in Aβ degradation to switch, leading to Aβ accumulation and plaque formation.
Over the years many lifestyle factors have been linked to Alzheimer’s disease and cognitive decline; these include smoking, low physical activity, poor diet, mental inactivity. Further, diabetes, obesity and cardiovascular disorder have also been linked to an increased risk of developing Alzheimer’s in later life [4]. No singular lifestyle risk factor has been proven causative and many are under debate; however, a healthy lifestyle has been shown to be beneficial for many reasons and is worth following.
 Understanding the role of APOE genotype on brain function
Understanding the role of APOE genotype on brain function
Researchers have investigated the relative effect on the brain of different genotypes of APOE, a known genetic risk factor for late-onset Alzheimer’s disease.
How is Alzheimer’s disease treated?
There is no cure for Alzheimer’s disease and treatment is predominantly symptomatic. For the non-cognitive symptoms, antidepressants and antipsychotics may be used to help reduce the mood and behavioral changes. Coping strategies are also provided to both the patient and their caregivers to help with the adjustment.
For those with early-stage Alzheimer’s disease, acetylcholinesterase (AChE) inhibitors may be prescribed to help alleviate cognitive symptoms. These are not recommended for those with late-stage disease. AChE inhibitors such as donepezil, galantamine and rivastigmine promote communication between cholinergic neurons in the brain by preventing the breakdown of the neurotransmitter acetylcholine. For those with severe disease, memantine may be prescribed; it is believed to act as an NDMA receptor antagonist, blocking the effects of the neurotransmitter glutamate and thereby reducing glutamate-mediated excitotoxicity.
What does the drug development pipeline look like for Alzheimer’s disease?
Alzheimer’s disease drug development has a high rate of failure and no new drugs have been cleared for clinical use since memantine in 2003. Since then, approximately 50 drug candidate compounds have passed Phase II clinical trials, though none have made it past Phase III [5]. However, research continues and there has continually been active trails and drug candidates in the development pipeline. In a review of the Alzheimer’s disease drug development pipeline for 2020 [6], it was found that 121 agents were currently undergoing clinical trial; this includes 97 agents for disease modification (delaying onset or halting progression), 12 for treating non-cognitive symptoms and 12 for preventing or reversing cognitive decline.
The authors found that research appears to be moving away from the amyloid hypothesis, with more emphasis on non-amyloid targets. Drugs targeted towards neuroprotection, reducing inflammation and epigenetic modifications were just some of the new candidates undergoing trial [6]. There has also been an increase in drugs being repurposed, a trend seen in many areas of drug discovery. One study found that 39% of drugs in the 2020 Alzheimer’s disease pipeline were repurposed from another therapeutic area, the most being hematologic-oncologic agents [7].
In 2013, world leaders came together and set a goal to have found a cure or effective treatment for dementia by 2025. Given the slow-paced nature of drug development, the goal set a tight deadline and given the relatively recent shift away from the amyloid-centric approach many of the drugs in the later stages of discover are still relying on a now controversial hypothesis. Indeed, the majority of Phase III clinical trials are on drug candidates that target Aβ, with only 15% targeting alternative factors [5]. However, the Phase II pipeline has a much more diverse range of targets, hopefully better reflecting the multi-factorial nature of the disease and therefore increasing the chance of success.
 Can a hormone replacement protect women against Alzheimer’s disease?
Can a hormone replacement protect women against Alzheimer’s disease?
Two-thirds of those with Alzheimer’s disease (AD) are women. With risk factors such as hormone levels and reproductive stage at play in postmenopausal women, researchers suggest an estrogen replacement may prevent Alzheimer’s onset.
Can Alzheimer’s disease be prevented?
There are currently no cures or prophylactic measures that can completely prevent the progression of Alzheimer’s disease. However, measures can be taken to reduce risk factors and potentially prolong the preclinical period of disease progression, delaying the onset of cognitive symptoms by a number of years [4]. Action area number three on the World Health Organization’s (WHO; Geneva, Switzerland) Global action plan on the public health response to dementia 2017–2025 is dementia risk reduction and in 2019, the WHO released their guidelines for risk reduction of cognitive decline and dementia.
Intervention strategies that are targeted towards improving modifiable risk factors, such as lifestyle habits and diet, have been demonstrated as viable for reducing the rate of cognitive decline. The first such study, the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) study, followed 1260 patients at risk of cognitive decline for 2 years and gave them multi-domain intervention strategies. Guidance included nutritional support, physical exercise programs, cognitive training exercises, planned social events and support for managing vascular risk factors. The randomized control trial found that those given support performed 25% better in neurocognitive performance tests after the 2 years than the control group. The FINGER model is currently being replicated in the USA, Europe, Australia and Singapore in a global study called World Wide FINGERS.