Bio-Rad Introduces Bio-Plex Pro Human IgG SARS-CoV-2 Serology Assays
The Qualitative Multiplex Immunoassay Panel Provides Precise, Specific, and Sensitive Detection of IgG Antibodies Against Four SARS-CoV-2 Antigens
HERCULES, CA, USA.–February 25, 2021–Bio-Rad Laboratories, Inc. (NYSE: BIO and BIOb), a global leader of life science research and clinical diagnostic products, today announced the launch of the Bio-Plex Pro Human IgG SARS-CoV-2 N/RBD/S1/S2 4-Plex Panel, for research use only (RUO).
The qualitative multiplex immunoassay kit quickly and efficiently detects IgG antibodies against four SARS-CoV-2 antigens, enabling vaccine developers to determine therapeutic efficacy from development through all clinical phases. In addition, the kit enables public health researchers to perform seroprevalence studies. This could help to identify the number of persons in a population who have been exposed to SARS-CoV-2 based on serology specimens, capturing individuals who have not received a COVID-19 diagnosis due to experiencing only mild symptoms or being asymptomatic.
“Detecting levels of antibodies against SARS-CoV-2 provides useful information about natural exposure rates in a population or about vaccine immunogenicity,” said Candice Cox, Bio-Rad Immunoassays Marketing Manager, Life Science Group. “We are pleased to be able to offer this new immunoassay kit to researchers working in vaccine development and related fields.”
About the Bio-Plex Pro Human IgG SARS-CoV-2 N/RBD/S1/S2 4-Plex Panel
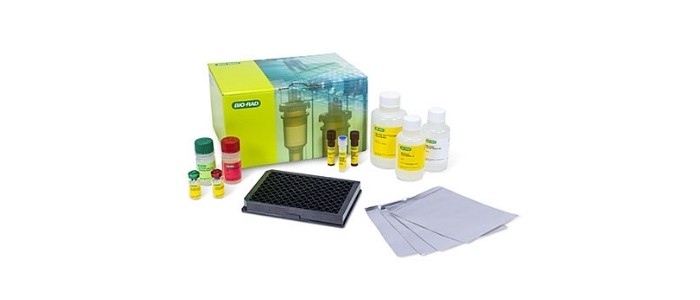
The Bio-Plex Pro Human IgG SARS-CoV-2 N/RBD/S1/S2 4-Plex Panel is a ready-to-use 96-well kit containing premixed magnetic capture beads, an IgG-specific detection antibody, positive and negative controls, and buffers that produce results for four anti–SARS-Cov-2 antibodies using only 5 microliters of sample. The offering includes singleplex assay reagents, enabling measurement of specific viral antigens of interest, and improves laboratory efficiency by generating key actionable data in a single experiment. The kit is compatible with RUO Bio-Rad platforms, including the Bio-Plex 200 and Bio-Plex 3D Multiplex Immunoassay Systems, as well as all other RUO xMAP platforms.
Key Benefits
- Helps researchers simultaneously profile antibodies against four SARS-CoV-2 viral antigens (nucleocapsid protein (N) and the receptor binding domain (RBD), spike 1 subunit (S1), and spike 2 subunit (S2) of the spike protein) in under 3 hours
- Confirms detectable IgG levels based on median fluorescence intensity (MFI) cutoff values
- Enables comparison of MFI values of samples to help researchers and vaccine developers understand both the strength of response and antibody levels at different time points after SARS-CoV-2 infection or vaccination
The coupled beads can be used to measure antibodies in any species, making the assay useful for both preclinical and clinical trials. For more information about the Bio-Plex Pro Human IgG SARS-CoV-2 N/RBD/S1/S2 4-Plex Panel and other Bio-Plex instruments, assays, and software, please visit bio-rad.com/Bio-PlexCOVIDAbs.
BIO-RAD and BIO-PLEX are trademarks of Bio-Rad Laboratories, Inc. in certain jurisdictions. The Bio-Plex Suspension Array System includes fluorescently labeled microspheres and instrumentation licensed to
Bio-Rad Laboratories, Inc. by the Luminex Corporation. LUMINEX and XMAP are trademarks of the Luminex Corporation. All trademarks used herein are the property of their respective owner.
This content was supplied by Bio-Rad.