Delivering genes across the blood-brain barrier
Adeno-associated viral vectors have been used for years to help deliver genetic cargo to different cells in the body. Now protein engineering is improving its performance.
Jude Samulski, one of the pioneers of adeno-associated virus (AAV) vectors, once likened the technology to a “molecular FedEx truck.” And for good reason: these small, genetically reprogrammed viruses were designed to help deliver genetic cargo to cells, be it a missing gene, a fluorescent label, or a therapeutic molecule. Yet, despite the method’s successes, these molecular FedEx trucks had difficulty getting past roadblocks such as the blood–brain barrier, and they often got lost traveling to more far-reaching cells, such as neurons in the peripheral nervous system.
Viviana Gradinaru from the California Institute of Technology (Caltech) wanted more tissue reach and clarity for her work on neurological disease. She believed that AAVs could be further engineered to help them reach neural tissue in both the brain itself and the peripheral nervous system.
“In order to see the circuits we wanted to study, we needed to be able to deliver the genes for visualization,” she said. “And once we started, we didn’t want to stop there. Because if you can deliver the genes for visualization, you can also deliver genes that might rescue a phenotype or give you new insight into how a particular mutation might affect the morphology of a cell.”
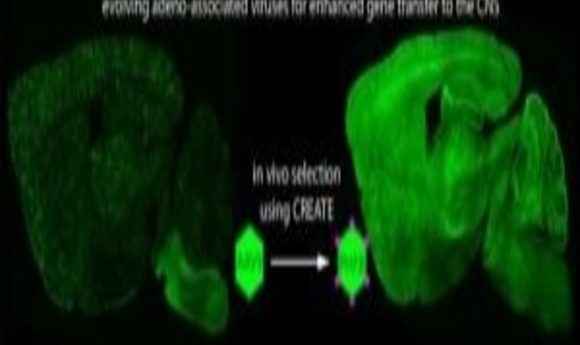
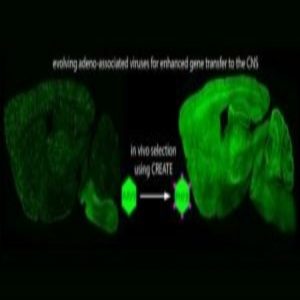
Working off of an AAV vector created in 2016, Gradinaru and colleagues genetically engineered the virus’ capsid, or external shell, using high-throughput protein engineering. The resulting viral vector proved able to cross the blood–brain barrier. Their engineering efforts also produced a second AAV variant capable of reaching neurons in more difficult-to-traverse regions, such as those found in the peripheral nervous system.
“The mechanism for crossing the blood–brain barrier is not known—which is why it is has been so hard to find ways to cross it with AAV. But when the mechanism is not known, you can design large libraries of variants of the reagents at hand,” she said. “We made millions of variants and then applied them to living mice. We designed the vectors in such a way that we could recover the sequences that crossed the blood-brain barrier or made it into a particular cell type. So, in a sense, we allowed the rodents to select which variants worked for us.”
While Gradinaru cautions that there is still a lot of work to do to validate this method before translating it into a potential treatment vector for neurological disease, she is optimistic. “There is a lot of promise in this approach. We were able to bypass the blood–brain barrier as well as get the vector into these different neural tissues across the nervous system,” she said. “And this is all possible with protein engineering.”