The molecular biology of social preference
A new study shows that neurotransmitters and miRNAs play a central role in social preference.
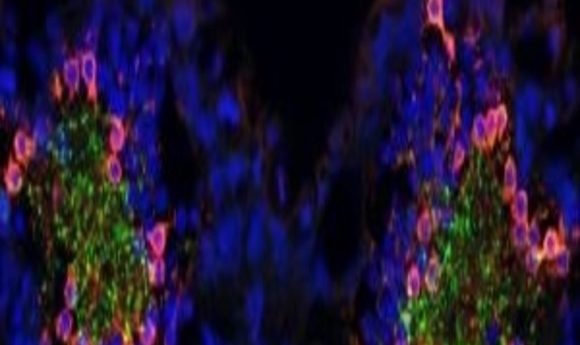
A confocal image of a tadpole brain reveals dopaminergic (green) innervation, mediating typical kinship recognition, and GABAergic (red) neurons elevated in cases of expanded social kinship. Nuclear marker in blue.
Credit: Davide Dulcis, UCSD.
African clawed frog (Xenopus laevis) tadpoles are attracted to siblings and repelled by non-siblings based on kin-specific chemical signals, known as kinship odorants. While researchers have been aware of this phenomenon for decades, little is known about the molecular neurobiology of kin preference. Now, a team of scientists has examined the brains of Xenopus tadpoles exposed to sibling and non-sibling odorants during development, revealing new insights into how kin preference may occur.
“Tadpoles are a great system for scientists like us that want to link molecular mechanisms to behavior in intact brains. Unfortunately most genetic tools for cell-type specific manipulation have been developed in rodents, therefore it’s very hard to dissect circuits in Xenopus,” said Davide Dulcis from the University of California, San Diego.
Dulcis’ team exposed tadpoles to non-sibling odorants, no odorants (orphans), or sibling odorants for two days. Next, they dissected the tadpole brains to look specifically for the neurotransmitters GABA and dopamine in the accessory olfactory bulb (AOB), which processes odorant signals.
“We see almost complete disappearance of dopaminergic interneurons and a staggering increase of GABAergic cells when animals were raised in the presence of non-kin odorants,” Dulcis said. In contrast, they found increased dopamine-producing neurons in tadpoles exposed to siblings and tadpoles with no exposure showed cells expressing both neurotransmitters.
The neurotransmitter changes occurred within the same neurons, suggesting that this process is dynamically regulated. “There is no cell loss or proliferation,” Dulcis said. “Neurotransmitter switching is a process of gene expression within preexisting differentiated neurons.”
Dulcis and his colleagues next confirmed that the neurotransmitter changes are relevant to kin preference by inhibiting GABA or dopamine in the AOB with drugs and observing a reversal of kinship preference. “Another surprising finding was that these changes in neurotransmitter identity would produce such a shift in behavior. The tadpoles exposed to non-kin odorants were now attracted to both siblings and non-siblings…a complete reversal of the natural behavior,” Dulcis said.
The team suspected that miRNAs might regulate neurotransmitter switching since miRNAs negatively regulate gene expression by targeting specific messenger RNAs for degradation and the switch in kinship preference correlated with a loss of either GABA or dopamine expression in the AOB neurons. After isolating miRNAs from tadpoles exposed to sibling or non-sibling odorants, the researchers found two microRNAs that target genes required for dopaminergic or GABAergic neuron identity; inhibiting either miRNA prevented the changes in GABA or dopamine marker expression following sibling or non-sibling odorant exposure.
Although Xenopus is evolutionarily very distant from mammals, Giordano Lippi , the project scientist and co-first author noted that miRNA regulation is well conserved: “Evolution went through a lot of trouble to preserve miRNA roles. Despite the obvious differences between tadpoles and humans, one can imagine that the mechanisms mediated by miRNAs, in this case switching of a neurotransmitter with another, are also highly conserved.”