It’s glow time: researchers develop fluorescent amino acid
Researchers have developed an unnatural amino acid that can be incorporated into existing proteins to create new fluorescent proteins, which will be valuable in the study of protein–substrate interactions.
Fluorescent proteins revolutionized the life sciences by enabling researchers to visualize biological processes; they are such valuable research tools that the 2008 Nobel Prize in Chemistry was awarded for the discovery of green fluorescent protein. Despite a multitude of fluorescence tools, there are still some biological processes that remain difficult to study, such as protein–metabolite interactions.
Now, researchers from Arizona State University’s School of Molecular Sciences and the Center for Molecular Design and Biomimetics in the Biodesign Institute (all AZ, USA) have developed a synthetic, fluorescent amino acid that can be incorporated into proteins to generate a number of new fluorescent proteins.
 Understanding the protein responsible for our ‘fight or flight’ response
Understanding the protein responsible for our ‘fight or flight’ response
As humans evolved, they developed a ‘fight or flight’ response to stressful stimuli in their surrounding environment. Researchers now believe that a regulatory protein found in skeletal muscle fiber has an integral role in this survival mechanism.
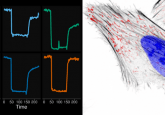
The study, published in Biochemistry, incorporated the fluorescent amino acid into the protein streptavidin, proximal to the binding site of its substrate, biotin. The fluorescence properties of the mutant streptavidin proteins changed when they interacted with biotin, a non-protein compound that is involved in a wide range of metabolic processes, including the digestion of carbohydrates, synthesis of fatty acids and gluconeogenesis.
“An important aspect of this study is that atomic-level pictures of many of these new proteins were generated that provide a great deal of information about how the binding of biotin changes the fluorescence properties of the proteins,” corresponding author, Jeremy Mills commented. “This information lays a foundation for the development of new fluorescent proteins that will help further the legacy that fluorescent proteins have already forged in the study of biological systems.”
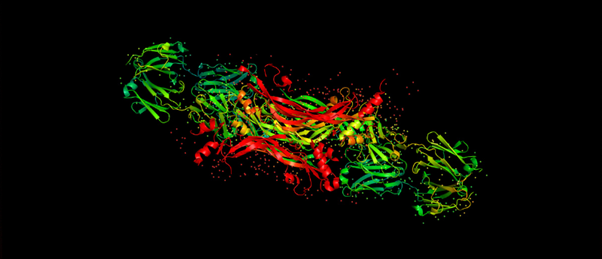
The researchers purified and crystallized the mutant streptavidin proteins in order to collect diffraction data. The data will be used in future studies aimed at the rational design of fluorescent, protein-based sensors of small molecule binding or dissociation.