Location of tumor cell alters its genetic activity
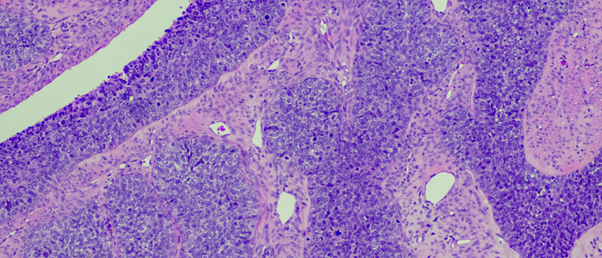
A new spatial single-cell transcriptomics method demonstrates that cells near the surface of a tumor have different genetic activity from those at the center.
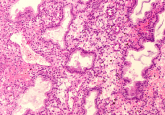
Two cells can be genetically identical but express different genes depending on their location and surrounding environment. In cancer, this means that tumors are comprised of cells that conduct different functions at different locations. To study this phenomenon, a collaboration co-led by researchers at the National Institutes of Health (MD, USA) and the University of Cambridge (UK) combined fluorescence imaging with high-throughput single-cell RNA sequencing to find out the impact of a cell’s location in a tumor on its gene activity. This could have implications for cancer treatment, for example in selecting the optimal drug combination to eradicate a tumor.
It remains a challenge to identify the spatial arrangements of cells in a tissue as they are often decoupled from their original position during analysis. Furthermore, some spatial techniques are limited in their ability to study 3D tissue architectures, such as multiplexed error-robust fluorescence in situ hybridization (MERFISH). The system developed in this study, called Segmentation by Exogeneous Perfusion (SEEP), overcomes these challenges by utilizing some ingenious technologies.
 Triplex origami folds double-stranded DNA with Hoogsteen interactions
Triplex origami folds double-stranded DNA with Hoogsteen interactions
Researchers have developed a method to program the spatial organization of DNA by incorporating Hoogsteen interactions.
First, a fluorescent gradient is induced in a 3D tissue model using a dye that diffuses through the model at a definable rate. The amount of dye detected by fluorescence imaging reveals how deep a cell is within the tumor model, providing a more accurate location for the cell. Second, single-cell RNA sequencing profiles their transcriptomes, followed by computational analysis, which allows the team to pair the cells’ gene expression profiles with their location.
The researchers used SEEP to analyze three types of lab models created from human ovarian cancer cells: spheroids, organoids and mouse models. To do so, tissue samples from these models were submerged in a bath containing the fluorescent dye, the cells are then barcoded, single-cell RNA sequenced and then computationally analyzed, to deliver spatially resolved single-cell RNA-sequencing data.
The SEEP method demonstrated that tumor cells near the surface were more likely to undergo cell division and turn on genes to protect them from the immune system than those located at its center. This validates previous understanding of the relationship between cell state and position within a tumor.
“It’s critical to understand that not every cell in a tumor will be exposed to a drug in the same way,” said co-corresponding author Tuomas Knowles (University of Cambridge). “A cancer drug might kill the cells on the surface of a tumor, but the cells in the middle are different and affected differently. That’s likely contributing to why some therapies fail.” Improving our understanding of how tumors are structured with techniques like SEEP could, therefore, lead to more effective treatment options for cancer patients and other diseases.