A dynamic, tuneable hydrogel for improved organoid generation

Researchers have developed a hydrogel that can be shaped while organoids develop, instructing them to form in a more comparable shape to their in vivo organ counterparts.
An international collaboration between the Zayed Center for Research (London, UK), itself a collaboration between Great Ormond Street Hospital (GOSH) and University College London (UCL), and the University of Padova (Italy) has developed a hydrogel that can be shaped and altered as the cells within it are growing. This breakthrough will allow the development of more accurate organoids that display a closer structural resemblance to the shape of the organs they are designed to mimic.
In the 10 years that have passed since the advent of organoid models, these 3D cell cultures have provided an inspiring new option for the study of basic and disease biology and for the assessment of novel drug candidates. However, while they have certainly invigorated the field, key challenges remain in developing truly representative models.
One particular challenge is getting the organoids to grow into the shape and structure of an organ, which is as important to organ function as its cellular makeup. Although creative solutions, such as magnetism, have been investigated to address this issue, primarily researchers attempt to resolve it by setting hydrogel scaffolds in a form that encourages the generation of a representative structure. However, during the in vivo development of organs, the tissue and environment surrounding it changes as it grows, as opposed to being preset, meaning that this approach has some severe limitations.
Setting out to resolve this challenge, the research collaboration looked back to their previously established 3D bioprinting technique that used a carefully formulated photosensitive polymer combined with Matrigel (a hydrogel system for organoid culture) to generate a hydrogel scaffold and conduct cell seeding simultaneously. This system used targeted irradiation from a two-photon microscope to cross-link the polymer, setting the hydrogel in shape.
Researchers at Hokkaido University have combined a hydrogel material with transplanted neural stem cells to grow brain tissue and report encouraging results in their mouse model study.
Adapting this system, the team was able to establish that their photosensitive liquid polymer could diffuse through a previously established hydrogel scaffold, which is then irradiated in specific locations to form a crosslinked hydrogel at key timepoints in the growth of the cells within the initial hydrogel scaffold. This live tuning of 3D hydrogel-in-hydrogel structures enabled them to guide the organoids to develop into specific shapes (Figure 1). With the system established, the team then set out to test it in four, previously challenging, organoid applications.
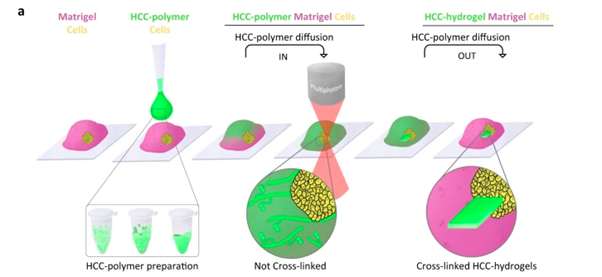
Figure 1: Strategy and set-up for hydrogel-in-hydrogel live bioprinting
Testing its ability to form complex structures, the team used this platform to grow intestinal organoids that replicate the structure of crypts and villi found within the intestines. Further, they established lung organoids that formed branches, just like in the development of a native lung. In a particularly informative case, the team created hardened gel ‘rails’ for neural organoids to grow along. This approach avoided the generation of disordered neuron bundles typically associated with previous organoid developmental approaches.
To highlight the utility of this approach in disease biology, the team tested it with cancer organoids, setting hardened gel cages around the cells and observing how the kinetics of the cells changed as they spread through hydrogel sections of different densities, mimicking the different tissues that cancers metastasize through.
The team hopes that by improving organoid models with this new technique, the movement away from animal models, recently accelerated by the FDA Modernization Act 2.0, will continue while improving the standard and translatability of cell culture studies. Further, this model could be used to investigate organ dysfunction due to malformation during development.
Extolling on the value of collaboration for this project, which reflects the growing trend in the scientific community to embrace support from international sources, co-lead author of the study Paolo De Coppi (UCL, GOSH) concluded that:
“This work is an excellent example of how we can bring interdisciplinary, international teams together to improve our research and benefit patients. Teams at GOSH and UCL that specialize in organoid research in the UK, working with Italian teams specializing in design and application of gel-printing, are what have made this incredible and beautiful piece of research come to fruition.”