Improving organoid mimicry and reproducibility
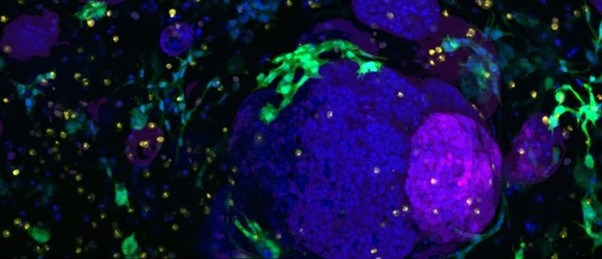
A new synthetic gel enables the generation of representative, reliable pancreatic organoid models of healthy and cancerous tissue and many other tissue types.
A recent collaboration between the Cancer Research UK Manchester Institute and MIT (MA, USA), led by Claus Jorgensen and Linda Griffith, respectively, has implemented a new protocol to produce pancreatic organoids. The method relies on a new gel that delivers a more reproducible and accurate model of both healthy and cancerous pancreatic tissue.
A better gel for better organoids
A recurrent issue in the development and implementation of organoid technologies is the variability of their composition due, in part, to the tissue-derived gels that are frequently used as scaffolds for their growth. According to Griffith, the most commonly used of these gels can vary from batch to batch and can also limit the number of cell types that can grow in the 3D culture.
To address these limitations, Griffith set out almost a decade ago to produce a synthetic gel that could support multiple cell types. After extensive research of the biophysical and biochemical properties of the extracellular matrix, which the gel acts almost as a stand-in for, Griffith incorporated key components of this matrix into a polyethylene glycol gel. Peptide ligands that interact with integrins on the surface of cells enabled cells to bind to the gel, while synthetic peptides derived from fibronectin and collagen permitted the growth of different epithelial tissues, complete with supporting stromal cells.
Capturing cancer
With her new gel firmly established, Griffith teamed up with Jorgensen to see if it could help resolve a common issue in pancreatic cancer modeling. When pancreatic tumor cells are excised from their native tissue, they can lose their cancerous characteristics, leading to inaccurate recreations of these tumors.
To see if her gel would successfully generate accurate pancreatic organoids, Griffith provided Jorgensen with a protocol for the gel’s production. Once Jorgensen had all the reagents required and began to apply the protocol, “…it just worked!” He exclaimed, noting how simple it was to derive pancreatic organoids from both healthy and cancerous cell lines. “I think that speaks volumes of how robust the system is and how easy it is to implement in the lab.”
Comparing these organoids to cancerous pancreatic tissue from mouse models, the team noted several similarities in the integrins expressed. What’s more, cells commonly found in the tumor microenvironment, including macrophages and fibroblasts, were also able to thrive within these new models.
Patient-derived pancreatic organoids
Looking at the translational capabilities of these models, the team assessed their ability to create patient-derived organoids – one of the most exciting contemporary applications of organoids – and were pleased to find that they were capable of the feat. Patient-derived organoids can be used to screen a specific therapeutic regimen to assess its potential for success in treating an individual patient; this represents a significant step towards a personalized medicine approach to cancer therapeutics.
With this capability validated, Griffith intends to develop patient-derived organoid models of endometriosis – a condition characterized by the growth of the tissue of the uterine lining outside the uterus.
As the gel used to create these organoids is synthetic, it can be created reliably using a simple workflow that yields a gel of the same composition each time. This makes methods reporting much simpler and therefore, the repetition of work by other labs much easier, allowing researchers to build on these studies and dramatically increasing the value of results derived from these organoids. With reproducibility constantly at the forefront of biomedical research, particularly in a new and emerging field, technologies such as this that increasingly standardize processes are in high demand.