Easy quantification of protein glycan phosphorylation

Researchers have developed a rapid and direct method for quantifying phosphorylation of protein glycans.
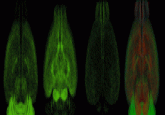
Protein phosphorylation, which is critical for their proper function and subcellular localization, can take place either directly on amino acid side chains or on the glycans of glycosylated proteins. Quantifying protein phosphorylation using current methods can be difficult, indirect, and time-consuming, especially for phosphorylated glycans. In the September issue of BioTechniques, Chikkathur Madhavarao and colleagues at the Food and Drug Administration describe a rapid and direct approach using simple lab equipment for quantifying the relative amounts of phosphorylated glycans and phosphorylated amino acid side chains in proteins.
In this new approach, the researchers separate protein samples by SDS-PAGE, and then stain the gel with the fluorescent phosphate-specific binding dye Phos-Tag, followed by a protein-binding fluorescent dye. Quantifying the binding of these two dyes provides the number of phosphates per protein molecule. Researchers can distinguish between phosphorylated glycans and phosphorylated amino acid side chains by treating the protein samples before gel separation with either peptide-N-glycosidase F (PNGase F) to remove N-linked glycans or with alkaline phosphatase to remove all phosphate groups.