CRISPR/Cas9 eliminates chromosomes
CRISPR/Cas9 has been used to generate cells or animals carrying precise gene mutations, including the rearrangement and deletion of chromosomal segments. Now researchers show that CRISPR/Cas9 can also remove entire chromosomes, opening new avenues for modeling and generating therapies for cancer and aneuploidy diseases.
Normal human cells have 46 chromosomes, and any deviation from this number, known as aneuploidy, is typically lethal. In humans, the most common aneuploidies are trisomies, which are characterized by the presence of one additional chromosome and represent about 0.3% of all live births. The most common human trisomies are Down syndrome, which is associated with an extra copy of chromosome 21, and Klinefelter’s syndrome, which is characterized by two X chromosomes and one Y chromosome.
Because each chromosome contains hundreds of genes, the addition of even a single chromosome disturbs the delicate balance of gene products in cells. While the past decade has witnessed major advances in strategies to correct single-gene defects of rare monogenic disorders, only a few attempts have been made to genetically correct the overdose of genes for an entire chromosome in aneuploid cells.
Now a study published in Genome Biology reports the use of the CRISPR/Cas9 system to eliminate targeted chromosomes (1). “Previous approaches to remove entire chromosomes from cells were laborious and very inefficient,” commented Kiran Musunuru, an associate professor of cardiovascular medicine and genetics at the University of Pennsylvania (PA, USA). “CRISPR/Cas9 offers a new way to do this.”
The CRISPR/Cas9 system depends on a single guide RNA (sgRNA) that targets the Cas9 enzyme to specific genomic regions to induce double-stranded DNA breaks, which are repaired by non-homologous end joining or homology-directed repair. Harnessing the cell’s own DNA repair machinery, researchers can add or delete pieces of genetic material or make changes to the DNA by replacing an existing segment with a customized DNA sequence. CRISPR/Cas9-mediated genome editing has been used to generate cells or animals carrying precise gene mutations, including the rearrangement and deletion of chromosomal segments.
“We already knew that CRISPR/Cas9 works very well at making cuts and introducing mutations into single genes. Here the authors have shown that they can use CRISPR/Cas9 in a simple way to make multiple cuts throughout a single chromosome,” Musunuru said. “By chopping up the chromosome, they cause cells to lose the chromosome altogether. The efficiency is far higher than anything observed before and enables the approach to work in living animals.”
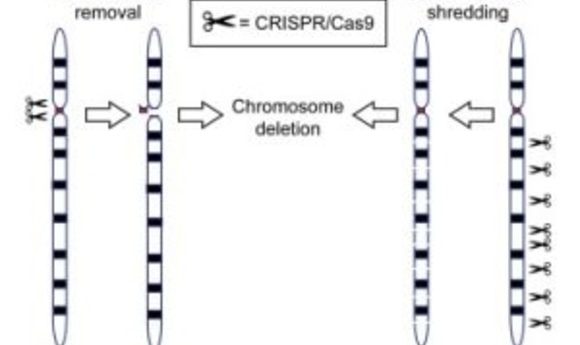
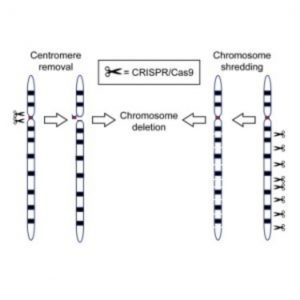
In the new study, the researchers used either multiple cleavages induced by a sgRNA that targets multiple chromosome-specific sites, or a cocktail of multiple sgRNAs, each targeting one specific site, to selectively eliminate a sex chromosome in cultured cells, embryos, and tissues in vivo. They also used this approach to produce a targeted autosome loss in aneuploid mouse embryonic stem cells with an extra human chromosome, human-induced pluripotent stem cells with trisomy 21, and cancer cells.
“CRISPR/Cas9-mediated targeted chromosome elimination offers a new approach in developing animal models and therapeutic treatments for aneuploidy,” noted senior study author Hui Yang of the Chinese Academy of Sciences (Beijing, China).
According to the authors, their study is the first to report X and autosome chromosome elimination via genome editing. Another study recently published in Molecular Therapy also demonstrated that the Y chromosome could be deleted in embryonic stem cells and zygotes by CRISPR/Cas9-mediated genome editing (2).
Far from the Clinic
The clinical application of CRISPR/Cas9-mediated chromosome elimination is not imminent, but the authors suggest that it has the potential to treat a broad spectrum of human tumors and even cure trisomies such as Down syndrome, Klinefelter syndrome, and XYY syndrome.
“You can imagine CRISPR/Cas9 is like a molecular scissor, which can specifically cut designated positions in the DNA sequence through the guide of sgRNA,” Yang explained. “Like a tie being cut hundreds of times with scissors, the chromosome would be destroyed beyond the limits of cell repair. Therefore, the targeted chromosome cannot participate in the process of DNA replication during cell division and will disappear in the offspring.”
Importantly, Yang and his team did not detect obvious off-target mutations or chromosome rearrangements in the chromosome-deleted mice and cell lines. However, before CRISPR/Cas9-mediated chromosome elimination could be used clinically, it will be important to evaluate potential off-target effects.
“This approach to chromosome elimination is a substantial step closer towards therapy, particularly for those diseases that do not require a high fraction of cells to be edited correctly,” said Richard Frock, a research fellow in genetics at Boston Children’s Hospital (MA, USA). “Making targeting 100% efficient while minimizing collateral damage will continue to be the goal for safely ameliorating genetic diseases.”
Broad Applications
In the meantime, CRISPR/Cas9-mediated chromosome elimination could be used to study how tumor formation might be affected by aneuploidy, which is a hallmark of cancer. Moreover, the approach could efficiently generate better animal models for aneuploidy diseases such as Turner syndrome, which is characterized by a single copy of the X chromosome.
“The important take-home message from the current study is that now it is possible to target the genome not just at the gene level, but also the whole chromosome,” explained Juan Carlos Izpisua Belmonte, principal investigator of the Gene Expression Laboratory at the Salk Institute for Biological Studies (CA, USA). “In the future, this approach could be used for the generation of chromosomal aneuploidy models that can mimic human diseases. Especially, this approach could help in generating animal models with chromosomal abnormalities, which so far has not been an easy task.”
It remains to be seen whether the findings are broadly applicable, according to Vinod Jaskula-Ranga, president and CEO of Hunterian Medicine (MA, USA), a CRISPR company focused on developing therapeutics. “One can imagine that the propensity for DNA repair after significant DNA damage differs significantly in different cells or organisms,” he explained. “What about chromosome elimination in the human haploid cell line?”
This concern was echoed by Christoph Bock, who directs the Medical Epigenomics Lab at the CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences (Vienna, Austria). “The main practical limitation of this method is that few cell types tolerate massive chromosome-scale deletions, and that cells from non-inbred species such as human may behave in unexpected and unphysiological ways when an entire chromosome becomes haploid as the result of a chromosomal deletion,” he said.
Another potential use of the technique would be to model evolutionary changes to chromosomes, Jaskula-Ranga pointed out. “One could see this approach being used in conjunction with gene drives, as elimination of the Y chromosome could result in loss of males and collapse of mosquito populations,” he said. “The applications for the technology could be wide, and although in the distant future, it’s interesting to speculate on the therapeutic potential.”