Cryo-EM: The imaging technique taking the study of disease mechanisms by storm
As new developments in cryo-EM make the technology easier to use, while providing higher resolution details of cell structures close to their native state, a flurry of publications have burst onto the scene, exposing the mechanisms behind a variety of disease areas, from ALS and cancer to COVID-19.
 For Bernhard Goetze, Director of Product Marketing in the electron microscopy (EM) business of Thermo Fisher Scientific (MA, USA), the past year and a half marks the beginning of his hard work coming to fruition. Bernhard has been driven over the past decade to provide researchers with cryo-EM workflows that don’t require a specialist to operate, knowing the technique could significantly improve research efforts if it were more widely used. But what are the advances that have made these publications possible, and what further impact could cryo-EM have in the study of disease?
For Bernhard Goetze, Director of Product Marketing in the electron microscopy (EM) business of Thermo Fisher Scientific (MA, USA), the past year and a half marks the beginning of his hard work coming to fruition. Bernhard has been driven over the past decade to provide researchers with cryo-EM workflows that don’t require a specialist to operate, knowing the technique could significantly improve research efforts if it were more widely used. But what are the advances that have made these publications possible, and what further impact could cryo-EM have in the study of disease?
Can you tell us a bit more about the cryo-EM space at Thermo Fisher and your work at the company?
I work for the division of Thermo Fisher that makes hi-tech electron microscopes for room temperature research in life sciences, which mainly includes cryo- (or cryogenic) EM. This covers both the transmission electron microscopy (TEM) world and small dual-beam or scanning electron microscopy (SEM). That’s my focus here.
Cryo-EM has led to some fascinating breakthroughs in a variety of disease areas recently. Can you provide some examples of these publications?
In the area of neurodegenerative diseases, our microscopes have been helping to resolve structures like the tau protein in Alzheimer’s and in Parkinson’s. The material used in those studies comes from patient tissue, allowing us to look at the real thing unadulterated.
There have also been breakthrough publications in understanding how amyotrophic lateral sclerosis (ALS) works. The collaborators of our study in Munich have seen, for the first time, the cellular mechanisms that cause cells to die in your brain when you have ALS. Inclusion bodies, identified a long time ago but poorly understood, have been shown to bend the endoplasmic reticulum, ultimately leading to cell death.
There is a study from the University of California San Diego (CA, USA) that has revealed how a genetic form of Parkinson’s disease is caused by a mutation in a protein called LRRK2. This protein is inhibiting the microtubular transport inside cells and especially neurons, essentially gluing up the intracellular highways – the microtubules. Preventing the cell from shipping proteins back and forth within itself results in cell death and degeneration of the neurons, causing Parkinson’s. This study was able to decipher how this mechanism works and resolve the proteins involved to the atomic level. Resolving the proteins is very important if you’re looking for drug targets.
In this case, now that the native structure of LRRK2 has been resolved, it may now be possible to design drugs that can help to disassociate the ‘glue’ surrounding the microtubules, freeing up intracellular transport.
Turning to cancer, there were brilliant studies in Nature and in PNAS that identified liquid–liquid base separations, essentially new kinds of organelles that work without membranes. Working from the example of proteasomes, they have shown how these membrane-less compartments are associated with the endoplasmic reticulum and degrade misfolded proteins in these compartments, which is very exciting.
What new attributes of cryo-EM have led to these discoveries?
We have been developing cryo-EM technologies with our collaborators in the scientific community for almost 10 years. Recently the technology has been transformed into several solutions that have completed the experimental stages in the research institutions of our collaborators. People all around the world are now able to use these new instruments to look inside cells. To do this, these new technologies cut windows into cells and then resolve their proteins. All proteins inside the cell, down to sub-nanometer – almost atomic – levels, can be resolved. This can also be achieved in cryotomography.
What you can see when you cut these windows into cells, quite literally using small dual beams, is the mechanisms of how cells work from the inside. This can be used to do basic research in cell biology, in neuroscience, in cancer, to reveal mechanisms and to provide deeper scientific insights into how cells function internally. To minimize any alteration from the cell’s native state, we fix the cells by flash-freezing them. There are no fixatives or staining agents. We are looking at the naked truth, so you can observe and understand the mechanisms that lead to certain diseases in a cell that very closely resembles the in vivo setting. This has applications in both basic science, understanding how cell division or motor transport works, for example, but can also reveal disease mechanisms. It is this application in the study of diseases that has led to a real surge in publications this year involving cryo-EM in various disease areas.
This increased resolution and the ability to view cells in a form that is very close to their native state has greatly accelerated the pace of a lot of research. This has saved a large number of research groups time and money, while leading to some of the top-flight publications that I have mentioned above.
 Eric Chen: imaging the atomic-resolution structure of coronavirus ‘spike’ protein
Eric Chen: imaging the atomic-resolution structure of coronavirus ‘spike’ protein
Thermo Fisher Scientific’s Eric Chen discusses imaging the atomic-resolution structure of coronavirus ‘spike’ protein with cryo-EM technologies.
You mentioned the development of the ability to resolve cellular structures to an atomic resolution. Is that the key change in cryo-EM and cryo-ET that has led to these breakthroughs?
There are two breakthrough developments in the technology. The first is the sample preparation. If you want to cut a window into a frozen cell, then you need to do so by using a gallium ion beam to cut the cell open and produce what we call a lamella – a 200 nm thick ice sheet of cellular material – this is done by using a cryo-DualBeam, gallium FIB-SEM. This has been experimental for a very long time, but now our Thermo Scientific Aquilos 2 Cryo-FIB cryo-DualBeam solutions have matured to be usable by non-experts. The level of automation, the ease of use, the reproducibility that we have achieved by making this a serial technology and a serial instrument has helped to produce more viable samples.
The next development involves the step that follows the production of the lamellas. The lamella is transported into one of our Thermo Scientific Krios G4 Cryo-TEM products, which are 300 kV TEMs. For this stage, the development of tomographic methods and software, alongside the community, has led to an increase in resolution. This increase in resolution is a result of two developments. The first is the perfection of sub-tomogram averaging: taking several hundred proteins found inside your data for a cell and averaging those to increase the resolution. The other is attributed to our Selectris filter technology and Falcon 4 direct electron detector, which both increase the resolution further to a level that is normally only known in single-particle cryo-EM. The combination of these developments pushes away the blurry curtains of the resolution limits that we experienced in the past.
Can you take more than one lamella from a cell?
We have two methods of preparing these windows into cells. The first essentially lances away everything from the cell, leaving behind one thin sheet of ice. That method would only yield a single sample from one cell. You have hundreds of cells on a sample carrier, however, that you can prepare one after the other. You can have a lawn of cells on the carrier and you can mill multiple lamellas in multiple cells at the same time, then you can shuttle that carrier into the TEM and acquire several tomograms from each lamella, creating a data set.
The other technology that we have been enabling with the Aquilos 2 specifically, is what we call cryogenic lift-out. To make these breakthroughs in medicine, you need bulk samples, for example biopsies from tumors. By freezing chunks of tissue using high-pressure freezing technologies, and then extracting a little ice-hockey puck of tissue, you can then cut out several lamellas. The problem is you cannot directly image those and shuttle them into the TEM, you need to isolate the lamella from that sample.
To extract the lamellas, typically 1-5 microns, we have a needle cooled to liquid nitrogen temperature. The needle is welded to the lamella, which is then cut out, and the needle is retracted carrying out the lamella. Once extracted, it is welded to a special sample carrier, all in the small dual beam of Aquilos 2. Now the next frontier is to perfect that so that this lift-out mechanism is as automated as possible, broadening the application even further, beyond cells and cell cultures.
What have been some of the challenges in developing these new products and capabilities?
The main difficulties have been in modifying the instruments to allow them to be cooled to liquid nitrogen temperatures. It sounds very simple, but you have to be really careful about vacuum levels and contaminations within the microscope. Any kind of residual molecules in the vacuum will condensate on your sample. You must be very careful that the lamella is not coated with kind of any contamination that is flowing around in the chamber. We have worked hard to make this as clean as possible and I’m pleased to say we have managed it successfully.
Developing the application itself – how to use the beam to best mill out the lamella – was also a challenge. If you put too much power in the beam you melt the ice or you form crystals within the ice, which then interferes with the acquisition.
We’ve been able to standardize this so much that we have very defined application schemes that people can replicate. These are applied in our automatic software that helps people to use the technology immediately, rather than fiddle around for months until they find the right parameters. A huge focus for us has been to ensure that these products can be used by researchers without the need for a second PhD in electron optics to use them.
We have standardized the entire workflow from our partners with Leica (Wetzlar, Germany), who do light microscopy, all the way to the big 300 kilovolt TEMs. This has hugely simplified the process of cryo-EM and cryo-ET, giving users the ability to focus on the science rather than the technology and how to make it work.
What impact has cryo-EM had on COVID-19?
There was a recent publication in Science, which revealed how SARS-CoV-2 is injecting its RNA into the cell. This happens through a very complicated pore that the researchers have been able to resolve and describe for the first time. By identifying this pore, which is a virus-borne protein, they have identified a prime drug target to block the replication of the virus once it has entered the cells.
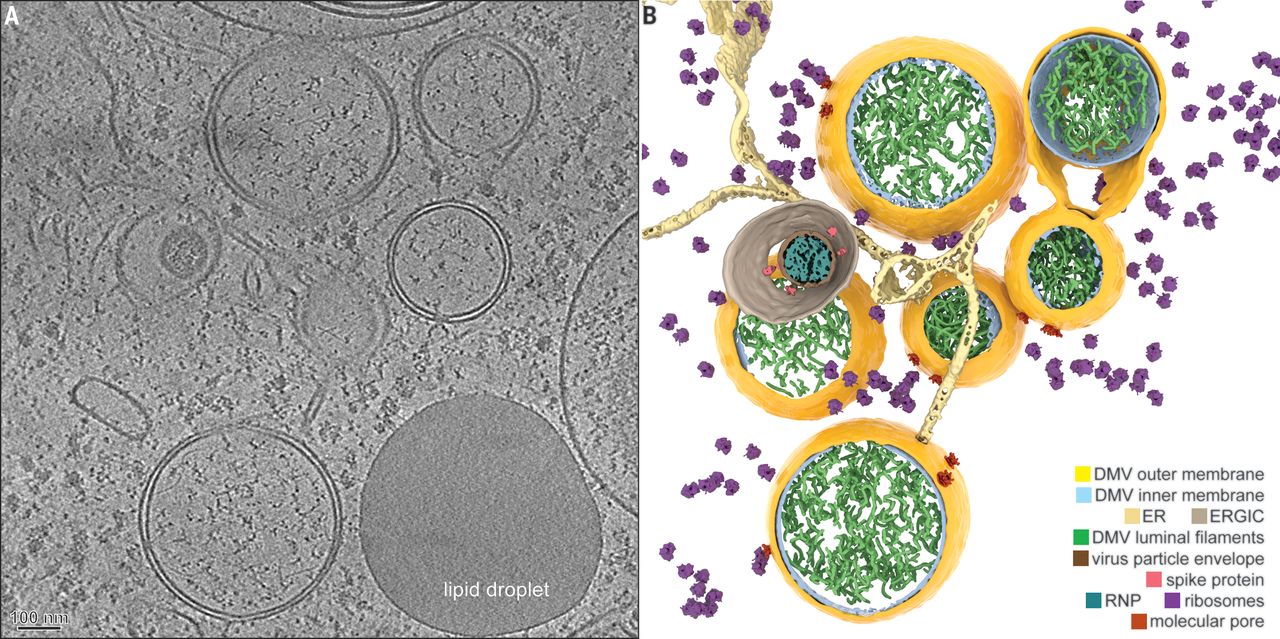
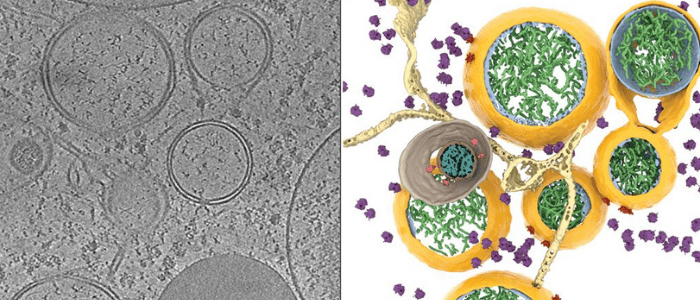
Coronavirus-induced DMVs revealed by cryo-ET [1].
Alongside this, the technique has played a role in vaccine development at the University of Oxford, Diamond Light Source, and the Rosalind Franklin Institute (all Oxford, UK). They have developed antibodies and vaccines based on these near-atomic structures and resolutions from both single particle cryo-EM and cryotomography. That shows the power and the speed improvement that this technology provides.
What further development would you like to see in cryo-EM in the near future?
Our focus is to make this technology available outside a very tightly knit cryo-EM community. Plans for four big cryotomography centers throughout Europe and Asia funded by the NIH (MD, USA) have been announced. A lot of people who already have our big cryo-electron microscopes, i.e. the TEMs, are adding cryotomography to their portfolio.
My personal goal is to bring this awesome technology to people who are traditionally using other methods like biochemistry, light microscopy and mass spectrometry to help us to better understand diseases.
I would be delighted to see cryo-EM deliver the same scientific excellence and insight that is has provided basic research to cancer researchers, neurobiologists, cell biologists, etc. enabling them to solve problems like neurodegenerative diseases which will cost society trillions of dollars in the next decade or two.
This interview was conducted in association with Thermo Fisher Scientific. To find out more about the cryo-EM solutions available from Thermo Fisher Scientific, visit: www.thermofisher.com/uk/en/home/global/forms/industrial/cryo-et-publications-e-book.html