The sugar key to unlocking T-cell memories
The paradigm of memory T-cell mediated immunity is shifting. Read about how researchers have defied textbook definitions.
Memory killer T cells traffic into non-lymphoid tissues in a process critical for protective immunity against infection; understanding these trafficking mechanisms will go a long way towards improving vaccine and immunotherapy design. Traditionally, scientists thought that protective immunity was mediated by a group of memory killer T cells called the effector memory (TEM) cells, but Jeffrey Nolz’s team at Oregon Health and Science University in Portland, Oregon recently published evidence in Science Immunology that challenges that notion.
Nolz initially set out to define the mechanism by which TEM cells entered into tissue. “I probably spent 3 years trying to prove that TEM trafficking was right, but I kept getting the opposite results. At some point you have to say, maybe the textbook isn’t exactly right,” said Nolz. He went on to find that central memory T cells (TCM) were primarily responsible for trafficking into non-lymphoid tissues.
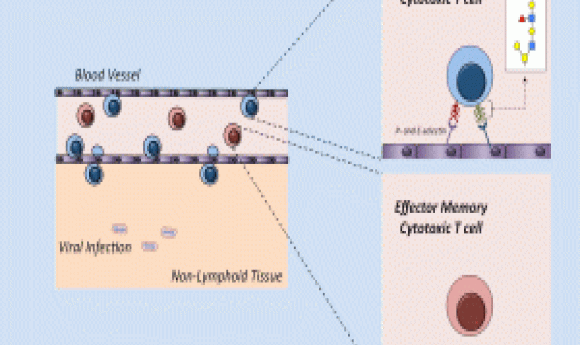
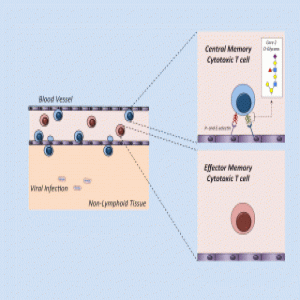
Previous T-cell trafficking studies only showed steady-state trafficking or distribution, but for this study, the researchers used a number of different models of inflammation, including intranasal challenges with inflammatory agents and viral infections of the skin. These types of inflammatory events trigger the expression of adhesion molecules on the associated vascular endothelium. Complex glycan molecules on the surface of immune cells bind to these adhesion molecules, enabling recruitment into inflamed tissue.
Nolz’s group identified a type of glycan molecule (core 2 O-glycan) synthesized on the cell surface of trafficked T cells, but this wasn’t necessarily a fundamental feature of all memory T-cell populations. “The capacity to synthesize these complex glycans is actually highly enriched on the central memory population of cells,” said Nolz. In further experiments, the team discovered that 2 O-glycan synthesis was turned on in activated central memory cells through both transcriptional and epigenetic regulation of glycosyltransferase enzymes responsible for core 2 O-glycan synthesis, thus providing a clear mechanism of action for TCM cells in killer T-cell mediated immunity.
“I think this will put central memory cells at the forefront of interest in the future, because it’s clear that those are targets for vaccination, and if we understand the paradigm that drives the trafficking for central memory cells, we will have a much better handle on it,” said Hermann Ziltener from the University of British Columbia, who provided the knockout mice used in this study, but was not otherwise involved in the work.
Nolz next plans to identify whether this type of glycosylation is a fundamental feature of trafficking in other lymphocyte populations and also to elucidate the long-term fate of cells after tissue infiltration. “We’re examining how these complex microenvironments (inflamed tissues) enforce subsequent long-term differentiation and are trying to understand the factors that contribute to longevity of the memory T-cell population,” said Nolz.