Most cited and most notorious: how the 2006 Alzheimer’s paper potentially misled research
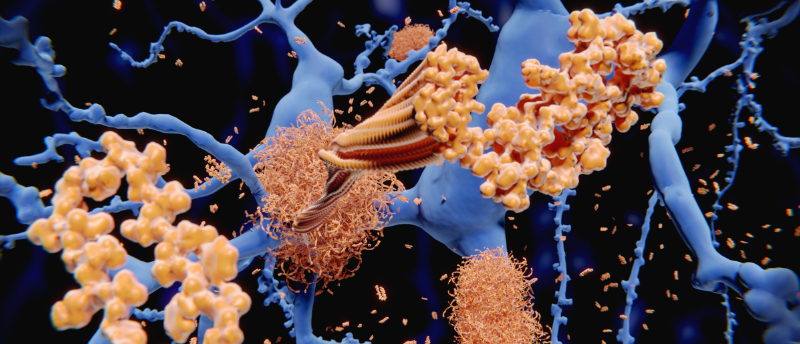
Data included in a pivotal Alzheimer’s study from 2006 may have been fabricated, potentially misleading research for the last 16 years.
Physician and neuroscientist Matthew Schrag (Vanderbilt University, TN, USA) raised concern about the validity of a research paper published in 2006 regarding the primary role of amyloid-beta (Aβ) plaques in causing the neurodegenerative disease, Alzheimer’s. This important paper served as the scientific basis for the development of an anti-Aβ plaque drug, called Simufilam, by Cassava Sciences (TX, USA).
Schrag’s own research had contradicted Cassava Sciences’ findings, leading him to investigate the drug for himself. It was in August 2021 that Schrag, then publicly unnamed, filed a petition with the US FDA to question the drug’s validity and safety, sending his work to the National Institutes of Health (NIH). By questioning this drug, Schrag opened an investigation that would be much bigger than initially anticipated.

Check out this Ask the Experts feature delving into the controversial recent approval of the Alzheimer’s drug, aducanumab.
During his technical and medical inspection of the drug’s scientific standing, he combed through a pivotal study from 2006 that detailed the discovery of an Aβ subtype that caused dementia in rats. One of the most cited studies about Alzheimer’s disease, this paper published in Nature by first author Sylvain Lesné (University of Minnesota Twin Cities, MN, USA) was found to contain apparently fabricated data and tampered images. Since its publication, this paper has informed and influenced the development of possible drug treatments and supportive studies. The NIH alone invested millions in the scientific research that supported the development of Simufilam.
Science launched its own 6-month investigation into the matter, conferring with experts in image analysis and Alzheimer’s. They agreed with Schrag’s conclusions that Lesné’s paper had likely reported false results. Molecular biologist and forensic image consultant, Elisabeth Bik, commented that the authors “appeared to have composed figures by piecing together parts of photos from different experiments. The obtained experimental results might not have been the desired results, and that data might have been changed to… better fit a hypothesis.” The investigation suggested that Lesné’s other research papers also contained fraudulent results; the University of Minnesota is reviewing these allegations.
Schrag’s discovery has massive financial implications. “The immediate, obvious damage is wasted NIH funding and wasted thinking in the field because people are using these results as a starting point for their own experiments,” commented neuroscientist, Nobel laureate and Alzheimer’s expert Thomas Südhof (Stanford University, CA, USA).
 eBook: An Alzheimer’s drug, from discovery to approval
eBook: An Alzheimer’s drug, from discovery to approval
Aducanumab was approved for use by the US FDA in June 2021, a controversial decision that caused much debate within the neuroscience community. This eBook serves as a timeline for the Alzheimer’s drug.
Recognizing what Schrag has uncovered, amyloid-hypothesis supporter Dennis Selkoe (Harvard University, MA, USA) hopes that “people will not become faint hearted as a result of what really looks like a very egregious example of malfeasance that’s squarely in the Aβ oligomer field.” In Selkoe’s view, although a hit to the amyloid hypothesis, the likely false results may not have misled research too much. The greater problem with this fabrication is that the public’s mistrust of science may increase, according to Selkoe.
Further research is essential to understand the role, if any, that Aβ plaques play in Alzheimer’s disease. It is equally important that this investigation serves as a reminder to think critically and act ethically in scientific research as it can, and often does, affect life. As Schrag says, “You can’t cheat to cure a disease. Biology doesn’t care.”
Want to keep up-to-date with more of the latest neuroscience news? Register for our weekly newsletter here and check out our neuroscience topic section here!