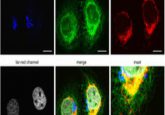
Ge Wang and Margarida Barroso on the HOTGEM imaging technology for breast cancer
BioTechniques spoke to Ge Wang (Rensselaer Polytechnic Institute; RPI, NY, USA) and Margarida Barroso (Albany Medical College, NY, USA) about their recent collaboration to develop a set of novel imaging techniques, known as HOTGEM.
Could you both please introduce yourself and let us know what you are currently working on in the lab?
 Barroso: I am a professor at Albany Medical College. I also teach on several international imaging courses and have two issued patents on FRET imaging technology. My group is a major force in the visualization, quantitation and identification of the mechanisms underlying the regulation of receptor-mediated endocytic pathways in cancer cells. I have established an international reputation as a FRET expert with unique capabilities of using advanced live imaging approaches to answer complex and important questions in receptor-mediated targeted therapy.
Barroso: I am a professor at Albany Medical College. I also teach on several international imaging courses and have two issued patents on FRET imaging technology. My group is a major force in the visualization, quantitation and identification of the mechanisms underlying the regulation of receptor-mediated endocytic pathways in cancer cells. I have established an international reputation as a FRET expert with unique capabilities of using advanced live imaging approaches to answer complex and important questions in receptor-mediated targeted therapy.
Recently, my laboratory has developed fluorescence lifetime (FLI)-FRET imaging to visualize and quantitate ligand-receptor target engagement. Furthermore, my laboratory and Xavier Intes’ group at the RPI established a strong collaboration to employ a non-invasive FLI-based imaging to enable real time in vivo information on receptor-ligand target engagement in deep tissues in live, intact animals.
In 2013, our group was the first to perform near infrared (NIR) macroscopy FLI (MFLI)-FRET in breast tumor xenografts in live, intact mice. In 2018, we demonstrated that MFLI-FRET measurements correlate with ligand binding to tumor cells, but strikingly, not with ubiquitously used ex vivo receptor expression assessment. The significance of this research lies in the ability to develop an online quantitative approach to measure target engagement in anti-cancer therapy, that will generate huge benefits for drug discovery and development.
 Wang: I am the Director of the Biomedical Imaging Center at Biomedical Imaging Center at RPI. I published the first spiral/helical cone-beam/multi-slice CT algorithm in 1991 and have since then contributed over 100 papers to theory, algorithms, and biomedical applications in this area of CT research.
Wang: I am the Director of the Biomedical Imaging Center at Biomedical Imaging Center at RPI. I published the first spiral/helical cone-beam/multi-slice CT algorithm in 1991 and have since then contributed over 100 papers to theory, algorithms, and biomedical applications in this area of CT research.
My group developed interior tomography theory and algorithms to solve the long-standing “interior problem” for high-fidelity local reconstruction, enabling omnitomography (‘all-in-one’) with CT-MRI as an example.
In 2016 I published the first perspective on deep-learning-based tomographic imaging as a new direction of machine learning, and our team published a Nature Machine Intelligence paper on the superiority of deep learning over iterative reconstruction. My research interests include X-ray CT, MRI, optical tomography, multi-modality fusion, and machine learning.
Could you please describe the nature of your collaboration and the aim of your study?
Wang: This project is a truly multi-interdisciplinary partnership between RPI, Albany Medical College and MARS Bioimaging (Christchurch, New Zealand), a tech company led by Phil Butler. Barroso, Butler, Intes and I are leading investigators in our respective fields, which are complementary for hybrid X-ray and optical tomography of small animal models, an area especially important for breast cancer treatment. More specifically, the overall goal of this project is to develop a hybrid X-ray and optical prototype for high-dimensional optical tomography guided by energy-resolved micro-CT (HOTGEM), which can be utilized to visualize and quantitate breast tumor heterogeneity, the expression and dimerization of HER2 as well as therapeutic responses in preclinical models.
Can you describe the novel X-ray imaging methods and technologies developed for this study?
Wang: With over 12 million New Zealand dollars of government funding from 2014 through 2020, Butler’s group is developing photon-counting CT technologies for preclinical and clinical applications. Wang is a major subcontractor of the current New Zealand grant and has been closely collaborating with Butler’s group for many years. Recently, the first photon-counting micro-CT scanner has become commercially available, developed by MARS Bioimaging. This novel technology generates multi-energy images with high spatial resolution for simultaneous quantification of soft tissues, contrast agents, nanoparticles and pharmaceuticals.
The scanner can differentiate up to six types of materials in a single scan, which is ideal for performing material-decomposition-based optical heterogeneity correction and contrast-enhanced cancer imaging. Optical molecular tomography can then be regularized by the background attenuation and tumor location priors.
In this project, we will customize the photon-counting micro-CT system into the HOTGEM system to upgrade whole-body preclinical imaging to the next level. Of relevance, our New Zealand collaborators have reported that the X-ray photon-counting detection technology has been scaled to humans. The fact that a large bore scanner based on the MARS photon-counting detection technology is ready for clinical trial dramatically increases the potential of our proposed preclinical HOTGEM system.
How will the combination of these techniques help monitor the response of cancer cells to therapies?
Wang: Optical imaging is unique in its capabilities of providing structural, functional and molecular contrasts from a wide array of light-tissue interactions. Functional and molecular optical imaging generates quantitative information of hemodynamics and multiple biomarkers simultaneously. Enhanced with lifetime imaging, we can monitor the microenvironment and protein-protein interactions.
Recently, one of the multiple principle investigators of this project Intes and his team members reported on a novel instrumental and algorithmic approach, which combines modern optics components and compressed sensing techniques, to enable the collection of high content 5D optical datasets (space, time, and spectrum). This new imaging platform, which was featured in Nature Photonics, is based on two main innovations. First, comprehensive whole-body imaging can be enabled via structured light illumination to acquire hyperspectral time-resolved data tensors so that functional (total blood volume HbT [μM] and oxygen saturation StO2 [%]) and molecular (fluorescence-based multiplexing, effective quantum yield, and lifetime) parameters can be quantitatively and tomographically mapped in breast cancer mouse models. Second, -a cutting-edge methodology has been developed based on a state-of-the-art Monte Carlo forward model for fast and accurate 3D reconstruction.
Overall, our novel optical instrumentation acquires in vivo data over the whole animal body with high throughput. From these datasets, quantitative images can be reconstructed for functional parameters, fluorescence probe distributions, and FRET signatures.
- Balkees Abderrahman on exhaled breath biopsy for cancer diagnosis
- Could a blood test for breast cancer be on the horizon?
- Sample, shower, shave: the at-home urine test that could revolutionize prostate cancer screening
Which therapies will you be testing these imaging techniques with and why?
Barroso: Recently, Intes and I utilized a MFLI-FRET platform to quantify ligand-receptor binding in breast cancer xenograft tumors in live intact mice. This approach is based on measuring FRET by estimating the reduction of the fluorescence lifetime of the donor fluorophore when it is within 2-10nm to one or more acceptor fluorophores. Most importantly, MFLI-FRET can discriminate soluble fluorescently labeled ligands from those bound by dimerized receptors. Hence, MFLI-FRET reports on molecular events at the macroscopic level in live, intact animals. MFLI-FRET imaging allows the use of any ligand/homodimer receptor as well as therapeutic antibodies such as anti-HER2 drugs used currently in the clinic.
Importantly, our results have demonstrated that MFLI-FRET measurements correlate with ligand-receptor binding in tumor cells but strikingly, not with receptor expression assessment. This is a critical issue in targeted drug therapy, as ex vivo receptor profiling is the main method employed in tumor diagnosis and therapy. Despite this, targeted drug delivery in vivo may not always correlate with target expression levels. Our ability to measure target engagement in tumors is crucial to accelerate the prioritization of the most efficient targeted anti-cancer therapy.
Wang: A strong correlation between tumor heterogeneity, drug resistance and clinical outcome suggests that tumor heterogeneity is key for targeted therapeutics and precision medicine. Analytical molecular tools, based on biopsy or surgical excision, are widely utilized to characterize tumor heterogeneity. However, these provide an incomplete tumor sampling, making in vivo imaging crucial.
Importantly, the tumor heterogeneity in a subset of HER2-amplified cancers is associated with reduced survival and breast cancer progression. In order to improve the clinical efficiency of targeted therapies, there are major gaps in understanding the interplay between HER2 receptor expression level, targeted drug delivery and tumor response. Thus, we must simultaneously acquire exquisite tumor’s features, physiological states and pathological biomarkers, which can currently only be, to a limited extent, obtained with histology/microscopy.
Our innovative HOTGEM system will characterize HER2 receptor expression and dimerization, structural details such as vasculature and functional parameters including total blood volume and tissue oxygenation levels in human tumor xenografts using human cancer cell lines or patient-derived cells. After validation using histochemistry and microscopy, we will have early response parameters for targeted HER2 therapy. The HER2 receptor dimerization heterogeneity mapping is a novel feature that can only be extracted non-invasively using HOTGEM. Therefore, HOTGEM may provide a high predictive value for HER2-targeted therapy in tumors carrying HER2 activating mutations.
Why are you testing this technique in breast cancer as opposed to other forms of the condition?
Barroso: These imaging technologies can be applied to a wide variety of cancers since they depend on the receptor-mediated targeted therapies and not on the individual cancer location. We have focused on HER2-positive breast cancer because of the HER2-targeted antibody drugs available in the clinic. These drugs can be labelled using different near infrared dyes as well as other contrast agents to visualize their binding to tumor xenografts in live intact mice, using the novel imaging technologies developed in this project.
My expertise in receptor targeted therapy in breast cancer will be utilized to demonstrate and validate HOTGEM applicability to HER2 cancer studies. We will demonstrate the utility of the HOTGEM multimodality imaging approach to characterize in detail the correlation between receptor expression and dimerization, tumor morphology and drug sensitivity in HER2 based tumor xenografts. Moreover, our expertise in the area of tumor cell biology, HER2 cancer and tumor heterogeneity will help guide the application of this novel platform to cancer therapy and diagnostics.
How do you see these studies and the imaging techniques affecting the field over the next 5 years?
Wang: In 2014, the first and only demonstration of fluorescence optical tomography guided by X-ray grating-based micro-CT – a cutting-edge micro-CT technique that provides exquisite soft-tissue contrast – was reported with interesting results but major drawbacks, despite increased soft-tissue contrast.
The system consists of separate subsystems, has a long acquisition time (over 20 hours), performs very limited optical measurements, and is only suitable for ex vivo X-ray imaging. In contrast to X-ray grating-based imaging, over the past several years X-ray photon-counting imaging techniques have been under active development and achieved impressive results.
A specific aim of our project is to produce images in terms of attenuation coefficients as a function of X-ray energy, which enables chemically specific material decomposition and is more suitable for hybrid X-ray and optical tomography. The proposed hybrid HOTGEM system will be a breakthrough for anatomical, functional and molecular tomographic imaging in small animals. There are numerous preclinical imaging applications, including but not limited to HER-2 related cancer imaging studies.
Upon completion of this 5-year project, the proposed HOTGEM system will have been validated to offer 50μm X-ray resolution for material decomposition, as well as 100μm optical resolution for target localization in co-registration within 30 minutes for each hybrid in vivo scan, demonstrated to be a breakthrough for tomographic HER2 imaging. By then, HOTGEM will be ready for technology transfer and commercial translation.
Barroso: Recently, a more complex view of HER2 cancer has emerged due to widespread genomic sequencing and increased understanding of tumor heterogeneity. Therefore, the combination of these imaging modalities into an integrated non-invasive pre-clinical approach is crucial to further our knowledge of drug kinetics in cancer microenvironments, as well as our ability to assess early drug responses of breast cancer.
I am enthusiastic about the potential impact of this instrument that can integrate the ability to measure metabolic tumor levels with HER2-targeted and vasculature imaging to reveal target availability and accessibility. HOTGEM is a significant breakthrough for tomographic HER2 imaging in small animals and may provide high predictive value for HER2-targeted therapy in tumors.